INTRODUCTION
Endoscopic-based techniques in dry (Destandau, Arthrospine) and saline medium (percutaneous endoscopic lumbar discectomy [PELD], unilateral biportal endoscopy) are excellent methods for minimally invasive surgical treatment for symptomatic lumbar disc prolapse. One of the drawbacks observed with dry techniques is frequent blood staining of the scope lens tip when working close to the surgical target. This frustrates the surgeon and also adds to increased operative time. Excessive bleeding or accidental dural injury while working in saline endoscopy may necessitate conversion to an open/ microscopic technique. To address this problem, we modified and designed the second-generation Arthrospine Duo system which can be used in both air and/or saline medium. There is no such study about a single portal dual mode dry-saline endoscopic spine system utilizing both dry and saline medium for the lumbar discectomy technique reported in the literature. The objective of the current study is to describe innovative instrumentation, operative technique, and results in patients who underwent discectomy by Arthrospine Duo system for lumbar disc prolapse.
MATERIALS AND METHODS
From January 2016 to December 2016, 80 patients suffering from prolapse lumbar intervertebral disc were operated on by the Arthrospine Duo system. Prior ethical approval was obtained from ethical committee of the Trinity Hospital and Medical Research Institute, Zirakpur (Ref Number 1/2015).
1. Inclusion Criteria
Patients with single-level lumbar disc prolapse with unilateral radiculopathy with good clinical and radiological correlation.
2. Exclusion Criteria
Patients with bilateral symptoms, more than 1 level, double root involvement, cauda equina syndrome, and complete or partial foot drop and whose clinical symptoms did not match the magnetic resonance imaging (MRI) picture.
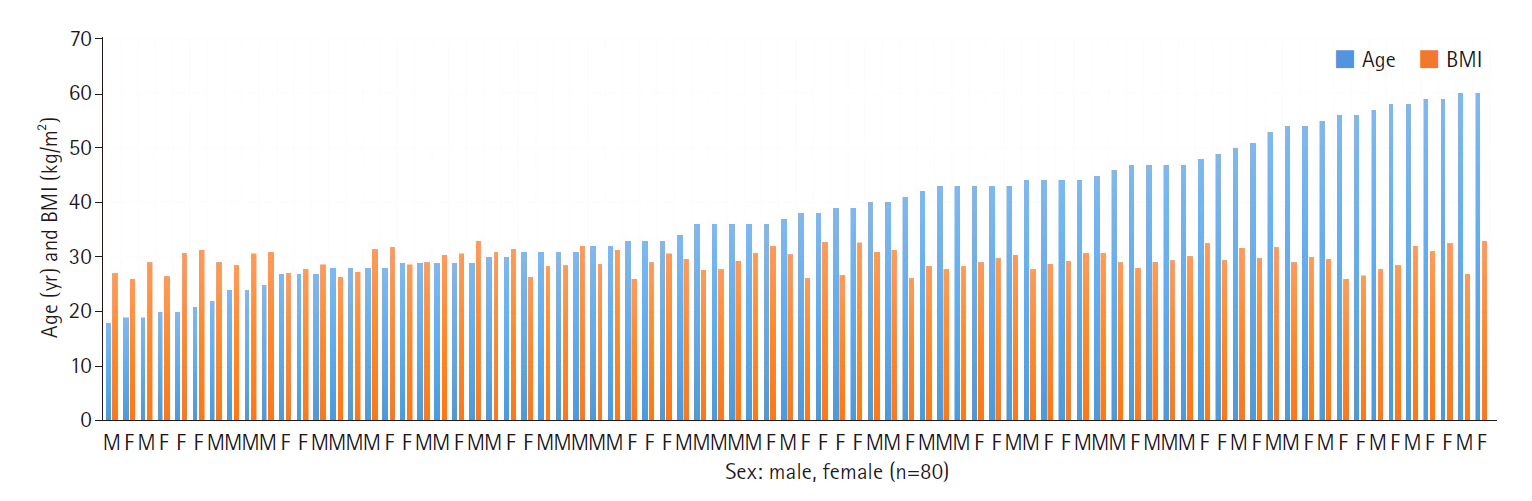
Interlaminar approach for endoscopic discectomy by Arthrospine Duo system (GESCO Healthcare, Chennai, India) was performed in patients who did not respond to medicines and physiotherapy for duration of 3 months. There were 45 males and 35 females aged between 18 and 60 years (mean, 38.4 years). The body mass index ranged from 26.1 to 33.0 kg/m2 (mean, 29.49 kg/m2) (Figure 1). The delay between the onset of symptoms to surgery was between 3 months to 1 year. Levels operated upon included L1–2 (n=1), L2–3 (n=1), L3–4 (n=4), L4–5 (n=69), and L5–S1 (n=25). Forty-five patients had radiculopathy on the right side and 35 on the left side. There were 58 paracentral, 10 central, 10 sequestrated, and 2 extraforaminal herniations. Average blood loss was 30 mL (range, 20-50 mL). There was no loss to follow up.
Written consent for lumbar endoscopic discectomy, anesthesia, pre-, intra-, and postoperative photography, and video documentation were taken for all patients. Clinical outcomes were analyzed using the modified MacNab criteria [1] and visual analogue scale (VAS) on postoperative day 2 and at the final follow at 2 years [2]. Patients were followed up maximum of up to 2 years duration. Out of 80 patients, 70 patients were given spinal anesthesia and 10 patients opted for general anesthesia. The postoperative protocol involved the mobilization of patients once the effect of anesthesia was over. Back exercise program and posture care were also taught at the same time. The rehabilitation program was altered in patients with unusual pain responses and dural tears. The patients were discharged on 2nd postoperative day. Postoperative follow-up was carried out on the 2nd, 6th, 12th, and 24 months. Patients were advised to remove water impermeable dressing on 3rd day and to keep the wound open thereafter since there were no sutures outside so these patients were not called for suture removal. They were only advised to report back in case there was any kind of drainage from the wound, fever, backache, or recurrence of sciatica. Back movements, neurology and straight leg raise were tested on every visit. During every follow-up visit, subjective perception of back and leg pain, work ability, neurological deficits, the need for analgesics, and the ability to return to work were analyzed. Postoperative MRI was only ordered in patients who had not shown satisfactory response to surgery or we suspected recurrence (Figures 2 and 3). Patients were followed up for a maximum of up to 2 years duration.
3. Arthrospine Duo System Assembly
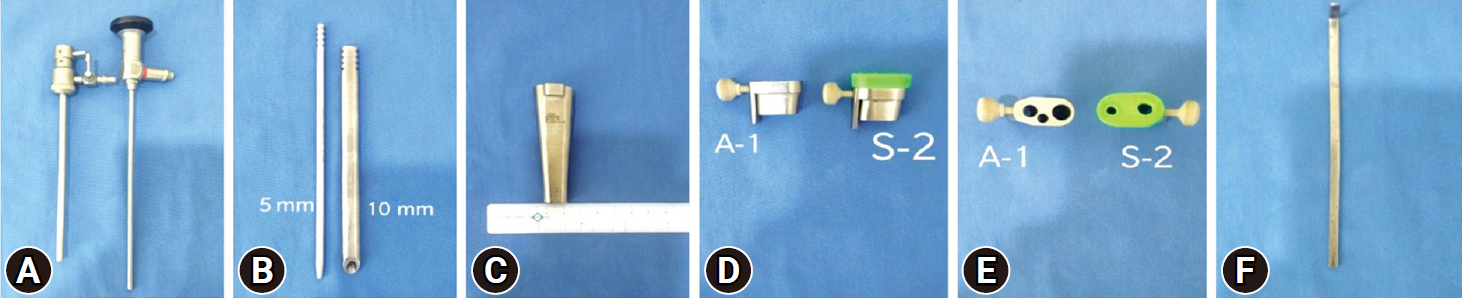
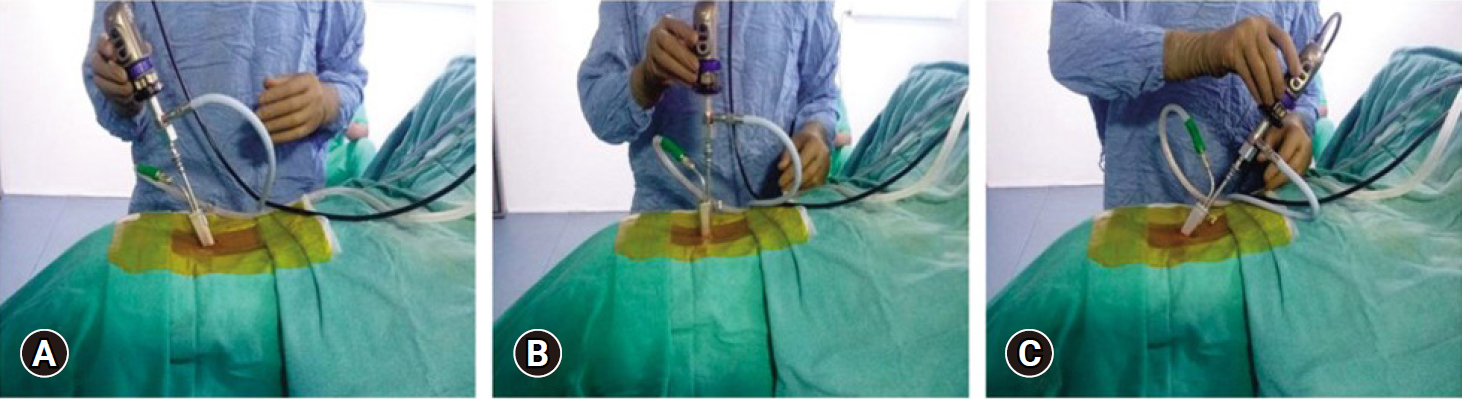
Arthrospine Duo (GESCO Healthcare, Chennai, India) system assembly comprises of single inflow cannula sheath which is compatible with 0° or 30° 4-mm arthroscope, set of 5-mm and 10-mm 2 cannulated dilators, 10-mm dilator has specially designed cobbs type tip to aid in soft tissue retraction from interlaminar window, Arthrospine conical oval tube 7 cm in length with lower channel of 12 mm2×8 mm2 and upper channel of 20 mm2×8 mm2, 2 Arthrospine working inserts air (A-1), saline (S-2) with a provision of tightening screw, working insert air (A-1), has 3 ports—first 6-mm port for scope sheath, second 4-mm port for suction cannula, and third 7.5-mm port for working instruments. Working insert saline (S-2), is covered with a silicone rubber cap and comprises two 5-mm ports, one port for passage of scope with sheath and another for passage of surgical instruments (Figure 4A–F). The saline enters through the single inflow sheath which is connected to the saline insert (S-2) which sits over the Arthrospine tube. The fluid enters into the operative field to create a hydrostatic pressure at the working area and continuously exits out of the second port of (S-2) insert which acts as a working port for inserting instruments (Figure 5). Radiofrequency equipment is an additional requirement for saline endoscopy for ablation and coagulation of tissues. We used the VAPR VUE (DePuy Mitek Inc., subsidary of Johnson & Johnson, Raynham. MA, USA) radiofrequency device, which can also be used in biportal and uniportal endoscopic surgery. Integrated nerve root and dural retractor are used for dural and nerve root retraction. The system is compatible with 0° and 30° 4-mm arthroscopes. However, we recommend a 30° arthroscope as it has a wide-angle view and enables better recess visualization. The tightening screw on the left side of the working insert allows the sheath and scope to be moved up and down, scope rotation clock or anticlockwise, and locked at the desired position. A discoscopic view is possible by negotiating the scope into intervertebral disc space in the saline medium. The system is handheld and mobile. The surgeon's left-hand controls the device and can tilt the system cephalad, caudal, medial, and lateral and can rotate clock and anticlockwise which enables the surgeon to navigate in the spinal canal including recess all around the dura and nerve roots (Figure 6A–C).
4. Operative Technique
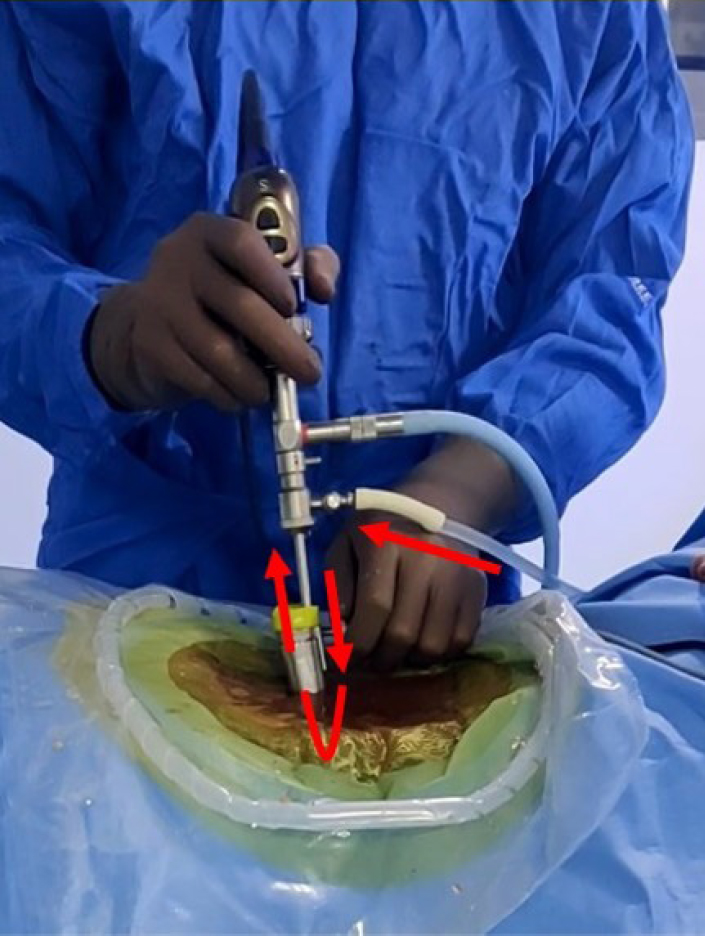
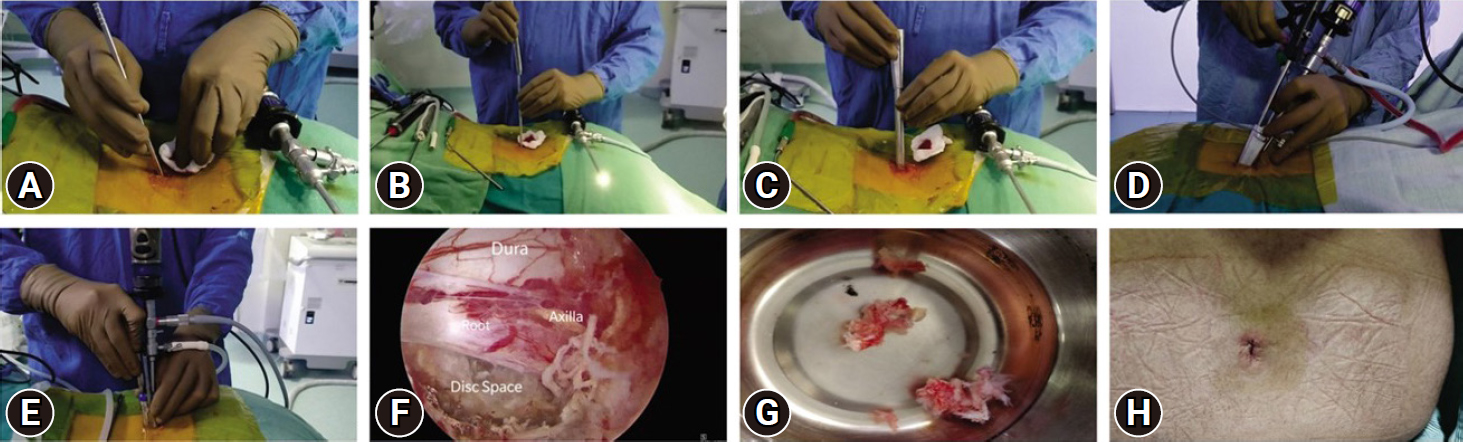
With the patient in a prone position over bolsters, the back of the patient is cleaned and draped. After administration of spinal or general anesthesia symptomatic lumbar level to be approached is confirmed using lateral fluoroscopy by inserting an 18-gauge spinal needle into the paraspinal musculature approximately one finger-breadth (1.5 cm) lateral to the midline. The needle is directed laterally towards the facet (to avoid inadvertent dural puncture) and repositioned until it is directly in line with the disc space. The spinal needle is then withdrawn and a 10- to 15-mm-long skin and fascial incision is made at the puncture site. Through this incision, a 5-mm dilator is inserted transmuscular towards the spinolaminar junction under tactile and fluoroscopic control (Figure 7A), followed by passage of a second 10-mm cannulated dilator with special cobs type tip over first dilator (Figure 7B), retraction of muscles and fibromuscular tissue from spinolamina junction, interlaminar window up to facet is achieved by 10-mm dilator. However, care must be exercised to prevent advancing the initial dilator into the spinal canal. The Arthrospine tube is introduced over the dilator over the symptomatic level then both dilators are withdrawn (Figure 7C). Arthrospine working channel air (A-1) is then snugly fit over the Arthrospine tube by simple press-fit way. The arthroscope is locked in the sheath and is connected to the endoscopic camera under sterile conditions. Scope with sheath and suction tube are introduced into their respective ports (Figure 7D). At this stage, the correct placement of the Arthrospine tube is checked under image intensifier guidance, to prevent wrong level entry in both anteroposterior (AP) and lateral views. For saline endoscopy, fluid comes out through a specially designed forward flow single portal endoscopic sheath. This fluid enters through one port and creates hydrostatic pressure at the surgical field which helps in tissue retraction and controls hemostasis and comes out through another working port. This is gravity gravity-aided open fluid flow system and no pressure pump is used here (Figure 7E). A radiofrequency probe is used to control hemostasis and to ablate tissues in saline, where ever needed. Arthroscopic 4-mm burr can be used to burr lamina to aid flavum detachment. For central and paracentral disc herniations, an interlaminar approach was utilized. For extraforaminal or far lateral disc prolapse, tube docking is done lateral to isthmus/ pars and after removing the foraminal ligament and part of the superior articular process tip, discectomy can be carried out. Under endoscopic visualization, fibromuscular tissue bulging in the mouth of the tube is shrunk with microbipolar coagulation (dry mode) or radiofrequency probe (saline view), this is further aided by the removal of soft tissue by pituitary rongeur. Cottonoids can also be used over the lamina to push away the fibro-muscular tissue and clear the lamina. Once boundaries of the interlaminar window such as superior and inferior lamina, facet joint, and spinolaminar junction are clearly visualized then initial bone work is started with a 2- or 3-mm kerrison punch or arthroscopic 4-mm burr at spinolamina junction thus detaching flavum from under surface of upper lamina. This is followed by partial or complete excision of ligamentum flavum leading to exposure of the dural sac and nerve root. Once neural structures are adequately exposed, the endoscope is advanced further to magnify and enhance the distinction between dura, root, and extruded disc. As the scope tip goes closer, it is prone to repeated staining by blood in dry mode at this stage surgeon can switch over to saline mode which mitigates staining of the scope tip and ensures excellent visualization (Figure 7F). Once the nerve root has been identified, it is retracted using a nerve root retractor or putting a cottonoid lateral to the shoulder of the root. The epidural veins are coagulated by microbipolar in dry endoscopy or radiofrequency probe in saline endoscopy. Depending on the pathology - annulotomy, discectomy of free loose fragments can be carried out (Figure 7G). An angled probe can be used to retrieve up/ down migrated or medial fragments while minimizing neural retraction. The scope can be further advanced into the disc space, in saline medium, to better appreciate the intradiscal pathology. At the end of the procedure, hemostasis of the muscle layers is achieved by microbipolar or radiofrequency coagulation. The tube is withdrawn and the lumbar fascia is sutured using vicryl 2-0 suture followed by the closure of the skin in a subcuticular fashion (Figure 7H) followed by water impermeable dressing. No drain is used.
5. Statistical analyses
Statistical analyses were performed with GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA, USA). Continuous variables were presented as means±standard deviations. Repeated analysis of variance and Tukey multiple-comparison posttest were performed to compare the differences at 3-time points of VAS pain score. Differences among the 3 groups were found highly significant at a p-value of <0.05.
RESULTS
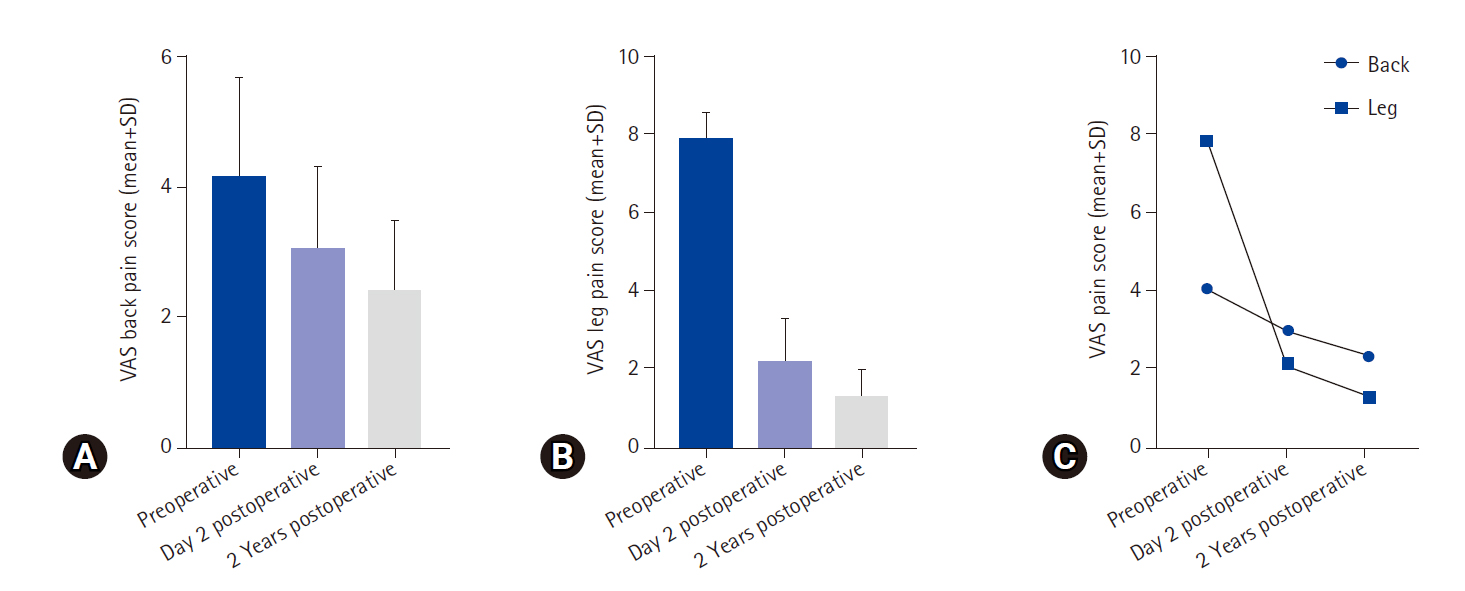
Clinical outcomes were assessed using modified MacNab criteria [3] and a numeric rating scale for back and radiating leg pain [4]. As per modified MacNab criteria, 64 patients (80%) had excellent, 10 (12.5%) good, 5 (6.25) fair, and 1 patient (1.25%) had poor results (Table 1). VAS numerical scale for leg pain improved from 7.87±0.68 to 1.3±0.67 at 2-year follow-up (Tables 2–4; Figure 8]. Average operative time was 45 minutes (range, 30–80 minutes). Intraoperative minor dural tears were observed in 3 patients (3.75%) which we managed by placing a gel foam over the defect followed by secure layered closure. They remained asymptomatic in the postoperative period hence rehabilitation protocol was not altered in these patients. They were told to report to the hospital if symptoms of giddiness, nausea, headache, fever, and cerebrospinal fluid leakage were observed from the wound site. Recurrent disc herniations occurred in 4 patients (5%) and underwent revision discectomy by Arthrospine Duo system. Nerve root injury occurred in 1 patient (1.25%) during dry medium endoscopy which contributed to poor results. Superficial delayed wound healing was observed in 5 patients (6.25%) which healed in 12-day time. These were managed and improved by regular wound dressings. All the patients were able to resume sedentary work in a week and routine activities 45 days after the procedure.
DISCUSSION
Laminectomy and discectomy advocated by Mixter and Barr [1] in the surgical treatment of prolapsed lumbar disc were associated with high morbidity hence many minimally invasive techniques were devised to reduce approach-related morbidity. Techniques such as chymopapain, percutaneous lumbar nucleotomy, transforaminal and automated disc removal devices [2,5–8], were minimally invasive but have not proven as effective as open lumbar disc surgery. The indications for these procedures have generally been limited to contained lumbar disc herniations. Bony or ligamentous pathology associated with disc herniation was a contraindication to these techniques. Microdiscectomy was introduced by Caspar [9] and Williams [10] and has been a gold standard. The disadvantage of these techniques, however, is, that the eye (lens of the microscope) is away from the surgical target, and the dissection of the short segmental paraspinal muscles (multifidi) from their bony attachments, can result in scarring as well as segmental denervation [11–14]. To further minimize approach-related morbidity to the spine, techniques by Destandau [15], Arthrospine [16], METRx [17–19], Full endoscopic [20], unilateral biportal endoscopy [21] have been successfully used through traditional posterior approach to treat all type of disc herniations. These authors have reported a success rate between 73% and 94%. Ruetten et al. [22] in 331 patients with lumbar disc herniation and minimum follow-up at 2 years found complete relief in 82% of patients. Only 13% had only occasional pain at the final follow-up. The recurrence rate was 2.4%. In another retrospective study by Choi et al. [23] in 67 patients with L5–S1 soft disc herniation treated with interlaminar PELD with more than 1.6 years of follow-up. Ninety point eight percent of patients showed favorable results. The mean hospital stay was 12 hours. The average time to return to work was 6.79 weeks. Complications included 2 cases of dural injury with cerebrospinal fluid leakage, 9 cases of transient dysesthesia, and 1 case of recurrence. Two patients required conversion to open procedure at the initial operation. Chumnanvej et al. [24] reported 91.6% excellent outcomes in their prospective analysis of 60 patients with 26 months of follow-up. Our results of the present study correlate well with the aforementioned studies. 80% excellent results in our study are comparable with those of Lyson et al. [25], Ranjan and Lath [26], Jhala and Mistry [27], Kaushal and Sen [28], and Oertel et al. [29], and other authors [30,31]. Notably, the complication rate associated with the present technique for lumbar discectomy is comparable with that of standard existing techniques. In the present study, dural injury and recurrent disc herniations were observed in 4 patients (5%), nerve root injury occurred in 1 patient (1.25%), superficial delayed wound healing in 5 (6.25%), and transient paraesthesias in 4 patients (5%). Recurrence rates of 5%, in our study, are comparable with Caspar [9], Williams [10], and Ebeling et al. [31]. These authors have reported reoperation rates of 5.5%, 5.7%, and 3%, respectively. The Arthrospine duo is a uniportal system that is used like a Destandau system in a dry medium and can be converted into a saline endoscopy medium by changing the top working insert. It is different from biportal surgery, which requires free hand control of both camera and instruments in each hand that requires a certain level of triangulation skill of the surgeon whereas the duo system utilizes a mobile conical tube which is controlled by one hand of the surgeon, inside which the triangulation is not needed as instruments and camera are coming in through the same tube. The system can be used in the anterior cervical approach in dry medium which is not possible with biportal surgery. Microendoscopic discectomy (MED) usually requires different sets of tubes that get fixed to the table henceforth lack of mobility in MED is a disadvantage. MED is done in a dry medium; biportal surgery is a fluid medium surgery. Compared to uniportal full endoscopy, the duo system does not require long, fine instruments which are prone to breakage and entail a recurring cost. A conventional bayonet Kerrison punch and standard arthroscopy burr can be used which is very economical in the duo system. The full endoscopic system requires different sets in cervical, lumbar, and dorsal levels whereas a single tube can be used in all levels in the duo system. The duo system can be used for multilevel degenerative spinal diseases for discectomy, and stenosis decompression where a single incision can be used to address L4–5 and L5–S1 by angulating the tube. If bleeding in saline becomes troublesome, then controlling becomes relatively easier in the dry medium where the camera lens can be taken away from the field to afford a bird’s eye view, and the red-out phenomenon of saline gets changed to oozing in the dry medium which can be effectively controlled with gelfoam, hemostatic agents and/or microbipolar coagulation. A dural tear in a saline medium can be a problematic situation that needs conversion to either open or microtubular methods; but by changing to a dry medium in duo system, it can be attempted repair with small metal clips (anastoclips) or placement of autologous fat/muscle graft which can then be augmented with a fibrin glue; the latter will wash away in a saline medium surgery. Excess fluid usage complications like neck pain, headache, long segment epidural hematoma, seizures, blindness, and abdominal collection have been described in fluid medium surgery – the duo system uses a dry medium approach till the flavum in the midline is removed and then can be converted to saline medium to ensure safer lateral recess and contralateral decompression in fluid medium hence ensuring judicious fluid usage. The versatility of the present technique which none of the other techniques offer is its use in both dry and saline medium. The advantage of the duo system is that the drawbacks of repeated blood staining of the scope tip while working in depth during dry endoscopy are taken care of by switching over to a saline medium, which affords a clear distinction between neural and non-neural structures. Only the working tips of instruments are visible during this procedure, this further reduces the chances of neural injury. Unable to perform adequate interbody preparation and cage insertion through the duo system tube is a drawback that the surgeon can tackle by removing the tube and converting to biportal surgery where the initial incision can be used as a working portal and another portal can be created over the adjacent pedicle (on AP view) which will be used as viewing portal in fusion cases. We have not used the duo system in tumor surgery but few authors have used the Destandau system for extradural tumor resections [32–34]. The endoscopic anatomy and appreciation of structures usually changes in both dry and saline medium which requires orientation and getting used to in initial cases. To minimize the steep learning curve, short-term fellowships, hands-on cadavers, and training on models are strongly recommended.