INTRODUCTION
Myeloid sarcoma, or MS, is a rare extramedullary tumor consisting of immature myeloid cells [1]. It can manifest in patients with acute myeloid leukemia (AML), myelodysplastic syndrome, or chronic myelogenous leukemia. The incidence of MS in AML patients ranges from 2% to 10.4%, with most cases associated with AML FAB M2, M4, and M5 [2,3]. Certain cytogenetic abnormalities in AML, such as t(8;21), have been linked to a higher incidence of MS [4]. The overall prevalence of MS in any organ is 2.9% among all patients with acute and chronic myeloid leukemia (CML). Specifically, the prevalence of MS involving the spine is 1.0% among these patients [5,6]. We report an extremely rare case of intradural extramedullary MS n a patient with AML as a hematologic relapse.
CASE REPORT
In this case, a 23-year-old female presented with left upper limb pain, weakness in the left hemimuscles, numbness in all 4 limbs, and difficulty walking. The initial manifestation as cervical pain progressed to radicular pain of the sixth cervical nerve, resulting in weakness (4 out of 5 strength in most muscles) and numbness in the left upper limb. Additionally, weakness (4 out of 5 strength) was noted in various muscle groups of the left lower limb. Reflex examinations indicated hyperactive tendon reflexes, a negative Hoffman's sign, and a positive Babinski's reflex.
Of significance, the patient's medical history revealed a diagnosis of AML from 2019 to 2022, highlighting a pertinent health background. Furthermore, the patient had a known sensitivity to soybeans.
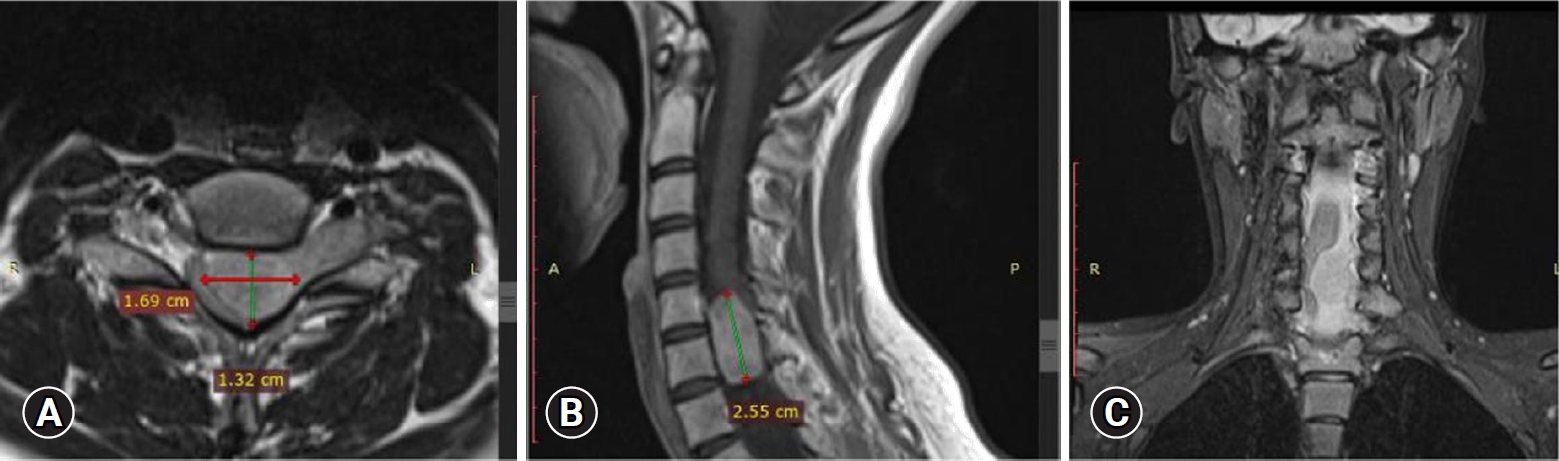
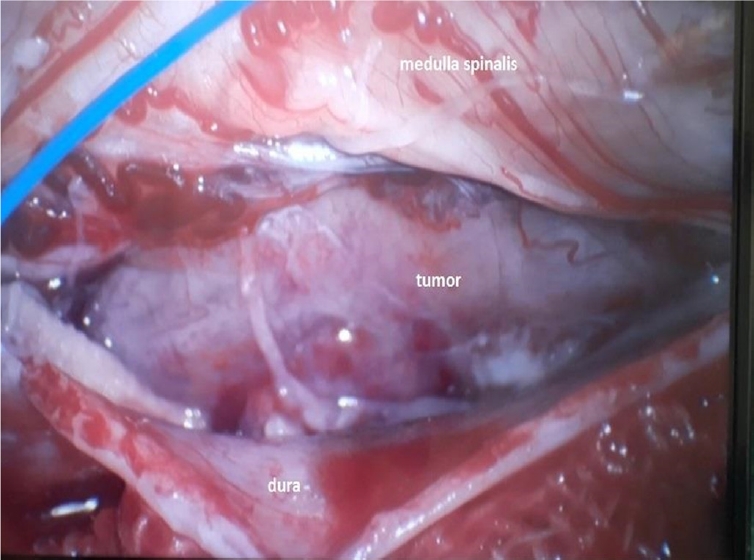
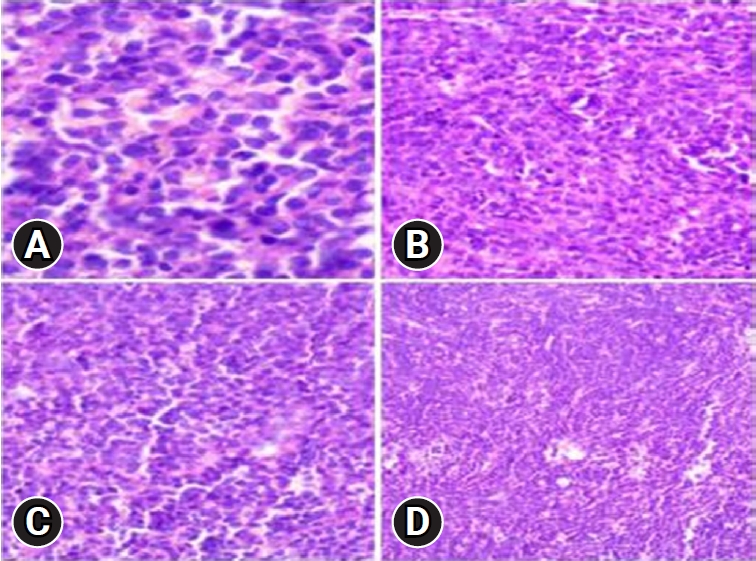
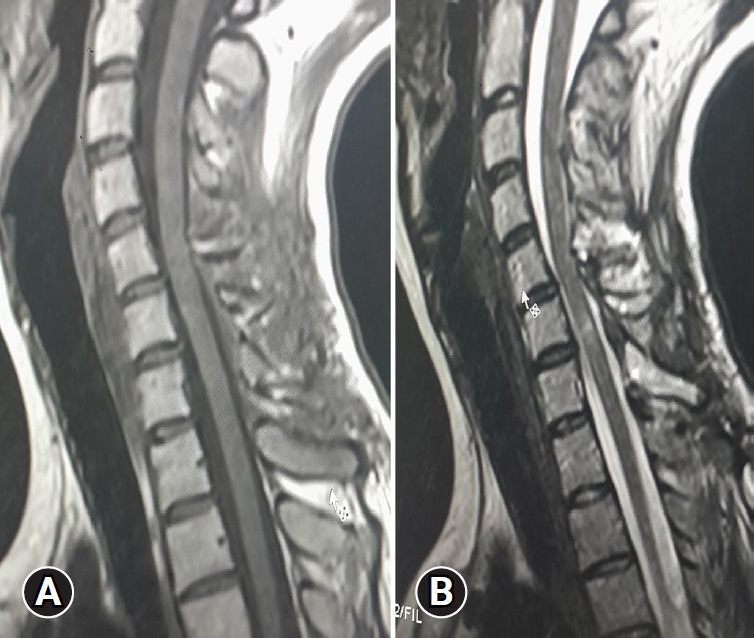
Upon admission, an enhanced spinal magnetic resonance imaging (MRI) revealed an intramedullary enhancing mass lesion, along with changes at the C5–7 level (Figure 1). The initial impression led to suspicions of granulocytic sarcoma or atypical infectious myelitis, prompting a consultation with the neurosurgery department for an open biopsy. The intervention commenced with a posterior midline incision, strategically chosen to provide optimal access to the cervical spine. The subsequent step involved a precise partial cut of the lamina, a critical maneuver to facilitate further exploration. The surgical field was then meticulously refined, as the dura mater, the protective covering of the spinal cord, was opened with utmost precision under the microscope. Following this delicate stage, tumor resection ensued with meticulous care, with a primary focus on minimizing damage to surrounding structures, particularly the nearby nerves. The surgical team executed laminoplasty using small titanium plates, securing them in place with screws to ensure stability and structural support. The spine, identified as intradural extramedullary with adhesion to spinal roots (Figure 2), was successfully resected without complications, surgical resection aspects were piecemeal with histological margins type R1, and followed by laminoplasty. After the operation, numbness and pain in the 4 limbs decreased, with improvement in pain in the left upper limb and 3 months after surgery the patient’s neurological examination was normal. Frozen biopsy results revealed malignancy with atypical small round cells (Figure 3).
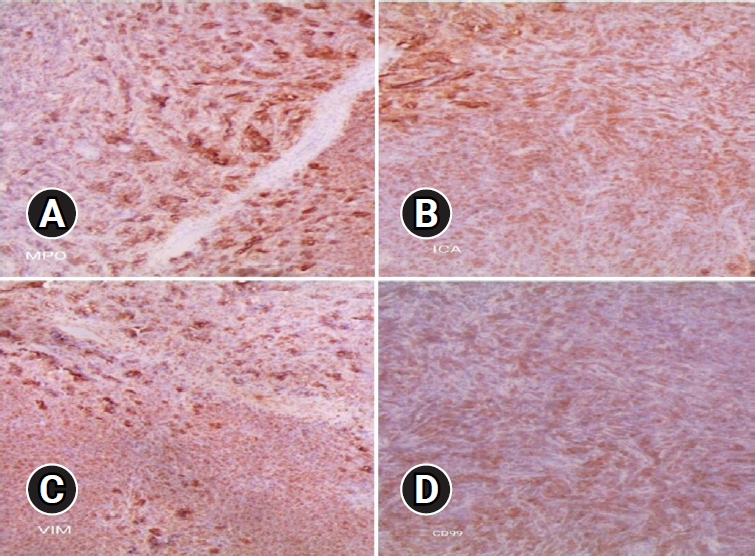
Final pathologic confirmation diagnosed the condition as MS, supported by positive immunohistochemistry for myeloperoxidase (MPO), leukocyte common antigen (LCA), and vimentin, and negativity for creatine kinase, desmin, synaptophysin, and CD99. Histologic findings indicated numerous blasts with round or oval nuclei, dispersed chromatin, prominent nucleoli, and agranular cytoplasm with varying basophilia. Eosinophilic granulation was notable in a minority of cell populations (Figure 4). Following histologic confirmation, the patient was transferred to the oncology department, and she underwent supplementary radiotherapy after surgery for 2 months and is currently under follow-up (Figure 5).
This case underscores the complexity of neurological symptoms in individuals with a history of leukemia and emphasizes the crucial role of comprehensive diagnostic procedures in achieving an accurate diagnosis for appropriate therapeutic interventions.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
DISCUSSION
MS, initially described in 1811, represents a rare hematologic phenomenon occurring in patients with myeloid leukemia [7,8]. Its prevalence in the spine is estimated to be less than 1% among all patients with acute and CML. While it can affect any level of the spine, it is more frequently observed in the lumbosacral spine, followed by the thoracic and cervical levels [9-11]. Clinical manifestations of spinal MS vary, depending on the tumor location and size [12-15]. Typically, spinal MS in the epidural or paraspinal tissues presents with signs of cord compression, resulting from a mass effect [14,16,17]. However, in some cases, as observed in the presented case, atypical intradural extramedullary location can lead to an unspecific presentation, potentially misdiagnosed as conditions like acute transverse myelitis or demyelinating diseases, leading to delayed diagnosis [8,9,13,18].
The diagnosis of spinal MS poses challenges as clinical and laboratory findings are often non-contributory [13,19]. Radiologic findings are usually non-specific, with the mass appearing as a soft tissue mass [16]. On MRI examination, spinal MS exhibits similar signal intensity to muscle on T1-weighted images and intermediate signal intensity on T2-weighted images, with subtle to intermediate enhancement with gadolinium [12,15,20]. Immunohistochemistry emerges as the most accurate method for confirming the diagnosis of MS, utilizing a series of markers for diagnosis confirmation and further lineage characterization [10,14,18,21].
MS can occur in patients without evidence of leukemia and may precede the development of systemic disease [13,14]. In cases where leukemia is established, the diagnosis of MS is relatively straightforward and should be considered in the differential diagnosis for patients with leukemia presenting with a soft tissue mass [21,22]. However, in the absence of a clinical history of leukemia, obtaining a pathologic diagnosis becomes crucial, as illustrated in the presented case, where repeated open biopsy and immunohistochemical stains aided in confirming the diagnosis.
The treatment strategy for MS hinges on systemic manifestations of leukemic cells and whether it represents the initial diagnosis or relapse [10,11,21]. The optimal timing and approach to isolated MS remain controversial. Delayed or inadequate systemic treatment often progresses to systemic infiltrations [15,19,21,23]. For patients with MS and concurrent marrow involvement, systemic treatment is recommended [13,14,18]. Prompt diagnosis and adequate systemic treatment are essential for a favorable outcome.
The differential diagnosis of MS in the head and neck region encompasses various malignant lymphomas, blastic plasmacytoid dendritic cell neoplasm, solid tumors (carcinoma and melanoma), and small blue cell tumors of childhood. It is crucial to distinguish MS from other entities, such as diffuse large B-cell lymphoma, Burkitt lymphoma, and lymphoblastic lymphoma/leukemia, emphasizing the significance of histological, flow cytometric, and cytogenetic analyses for accurate diagnosis.
CONCLUSION
MS, particularly in the spine, presents unique challenges in diagnosis and treatment. Timely recognition, accurate diagnosis through comprehensive evaluations, and tailored treatment strategies, considering the systemic manifestations and risk factors, are critical for achieving positive outcomes in patients with this rare hematologic phenomenon.