AbstractLumbar foraminal stenosis was suggested to exist as early as the 1800s; however, its importance faded when lumbar canal stenosis attracted attention. Subsequently, it was warned that lumbar foraminal stenosis should be considered as a “hidden zone.” Additionally, the importance of distinguishing foraminal stenosis from canal stenosis was reaffirmed when investigating the cause of lumbar nerve root symptoms. However, this condition is now widely recognized after the development of imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI); nonetheless, the accurate diagnosis of lumbar foraminal stenosis remains challenging. Lumbar foraminal stenosis is most commonly defined as a stenotic lesion extending from the medial edge of the pedicle to the lateral part. Conventional imaging examinations mainly include radiography and myelography; however, these imaging methods are unable to clearly visualize lateral lesions. Therefore, there is limited literature on lumbar foraminal stenosis. Subsequently, selective nerve root injection and CT have become popular, with MRI being the main diagnostic modality. The development of sequences such as 3-dimensional MRI, oblique coronal MRI, and diffusion tensor tractography has improved the diagnostic performance of imaging examinations. Thus, a better understanding of lumbar stenotic lesions among spine surgeons, in combination with more accurate imaging examinations, is expected to improve the accuracy of diagnoses, which in turn will help enhance the quality of treatment.
INTRODUCTIONHistorically, when spinal canal stenosis attracted attention, lumbar foraminal stenosis was treated as an outer type of spinal canal stenosis and was not established as a separate concept. However, with improvements in imaging examination technology, lumbar foraminal stenosis has been recognized again, and it is now diagnosed and treated as a separate concept from spinal canal stenosis. Lumbar foraminal stenosis accounts for a higher proportion of degenerative diseases of the lumbar spine than previously known and is often encountered in routine clinical practice; however, its diagnosis is challenging at times. Particularly in L5 nerve root entrapment, it is crucial to precisely distinguish whether the etiology is L4/5 lateral canal stenosis or L5/S1 intra- and/or extraforaminal stenosis. In addition, the diagnosis is important because it affects the surgical strategy. Incorrect determination of the surgical strategy, i.e., determining whether the patient requires decompression of the lumbar spinal canal or foramina, or requires fusion surgery, may lead to treatment of an inappropriate lesion, which may also cause failed back surgery syndrome (FBSS), and worsen the surgical outcome.
This report reviews the literature and outlines the history, classification, and evaluation methods of lumbar foraminal stenosis.
HISTORY OF DIAGNOSIS OF LUMBAR SPINAL FORAMINAL STENOSISGowers was the first to suggest the existence of lateral stenosis in 1891 in the earliest report. Although the term lateral stenosis is not commonly used, observations of the vertebral bodies of older people have shown that stenosis of the intervertebral foramina can cause radiculopathy [1]. Putti [2] reported that nerve root entrapment occurs due to facet joint degeneration. Danforth and Wilson [3] reported compression of the fifth lumbar nerve in the interstitial space. Later, Briggs and Krause [4] reported that a surgical method called interval foraminotomy was performed clinically in 1945. However, when Verbiest [5] proposed the concept of lumbar spinal canal stenosis in the 1950s, attention was focused on the pathology of the spinal canal. Lumbar foraminal stenosis was classified as lateral spinal canal stenosis, according to the international classification of lumbar spinal canal stenosis by Arnoldi et al. [6]. In 1971, MacNab termed the lumbar intervertebral foramina as the hidden zone and reported that clinicians should be careful not to overlook lesions in this area [7]. The hidden zone has now been widely recognized after imaging examinations, such as computed tomography (CT) and magnetic resonance imaging (MRI), and selective nerve root angiography/block.
Reports of lumbar foraminal stenosis increased between 1989 and early 1990s, after the introduction of CT and selective radiculography/block [8]. Further anatomical studies of the intervertebral foramen have advanced our understanding of its pathology. Until now, lumbar foraminal stenosis was considered to be relatively rare, but due to the increase in case numbers and advances in diagnostic imaging examinations such as three-dimensional (3D) MRI, its frequency is now thought to be higher than previously reported, and it is prevalent in 8%–11% of lumbar degenerative diseases [8,9].
CLASSIFICATION OF LUMBAR FORAMINAL STENOSISAlthough the above-mentioned international classification has been widely used, some limitations exist: the diagnosis by lesion site and the cause are mixed in degenerative stenosis, the definition of combined stenosis is ambiguous, and there is a lack of literature on the concepts of ossification disease and degenerative scoliosis (Table 1) [6,10].
In modern theory, the distinction between the spinal canal and the intervertebral foramen is based on the pedicle. It is possible to distinguish between the spinal canal and inner edge of the pedicle. The foramina extends from the inner edge to the outer edge of the pedicle, and the extraforamina extends from the outer edge to the outer edge of the pedicle. However, there are cases in which radiculopathy is continuously observed from within the intervertebral foramen to outside the intervertebral foramen. Moreover, in some cases, it is not possible to clearly distinguish between intra- and extraforaminal radiculopathy.
Compressive radiculopathy of the intervertebral foramen (foramina) is also known as foraminal radiculopathy. Foraminal stenosis is defined as an entrapment-type radiculopathy due to stenosis of the nerve root canal in the intervertebral foramen and not due to lateral disc herniation or lumbar spondylolisthesis (Figure 1) [11].
CLINICAL SYMPTOMSForaminal stenosis is a unilateral lower extremity symptom of a unilateral nerve root. It is known to be more severe than lower extremity pain caused by spinal canal stenosis, and often occurs at rest [12,13]. There have been reports of radiculopathy, which is considered a dorsal root ganglion symptom, such as lower extremity pain aggravated by sitting or lying on the affected side, and Kemp sign. Moreover, in recent times, the number of patients with extraforaminal stenosis and mild leg pain has increased. This mild symptom occurs due to chronic compression of the fifth lumbar nerve, which is a peripheral nerve distal to the dorsal root ganglion. It is difficult to distinguish it from general lumbar radiculopathy due to lateral recess stenosis. Yamada et al. [12] found that the frequency of intermittent claudication was high; however, the frequency of leg pain at rest and increased leg pain when sitting was low. Nerve compression at the level of the spinal nerve distal to the dorsal root ganglion has a different clinical picture than intervertebral foraminal stenosis [11].
DIAGNOSTIC SUPPORT TOOLYamada et al. [14] analyzed 49 patients with lumbar intra- and/or extraforaminal stenosis at the L5–S1 level and 51 patients with lumbar spinal canal stenosis at the L4–5 level. No significant difference was confirmed between the 3 subjective and 3 objective items from the Japanese Orthopedic Association; however, there was a difference in the pain provocation test results (Kemps sign, p=0.040; Bonnet test, p<0.0001; Freiberg test, p<0.0001).
These results were used to derive integer scores from β coefficients and develop a simple clinical diagnostic tool. For each patient, all the applicable risk score values were summed to obtain a total risk score ranging from 0 to 20. The results of the receiver operating characteristic analysis showed that the cutoff value was 5 points, sensitivity was 75.5%, and specificity was 82.3 % (Table 2) [14].
CONVENTIONAL EXAMINATIONSRadiography can be used to capture dynamic images, making it possible to ascertain the situation when the patient is in a standing position. A study on the height of the intervertebral foramen could provide important information for diagnosis. A disc height of 4 mm and a foraminal height of 15 mm suggest foraminal stenosis [15]. Myelography is less helpful in diagnosing foraminal stenosis because insufficient contrast filling is not observed in the distal nerve root sheath. On the other hand, CT can detect bony foraminal stenosis, which is difficult to detect using MRI and is also important for surgical planning. The shape of the foramen itself is poorly visible in the axial CT image; however, the parasagittal reconstructed image, which includes bony and soft tissue windows, better defines the space available for the nerve roots. Suggestive findings on CT reconstruction include the presence of osteophytes originating from the posterolateral vertebral bodies or articular surfaces that extend into the foramen [15,16].
Diagnostic selective nerve root injection (SNI) helps identify the responsible nerve root, and nerve blocks relieve symptoms and make the diagnosis more definitive. Diagnostic SNI can safely and accurately detect lumbar radiculopathy and convince surgeons to operate at an initially suspicious but incorrect level of radiculopathy. In cases where MRI findings are equivocal, multilevel, and/or do not agree with the patient’s symptoms, a negative diagnostic SNI result (i.e., lack of radiculopathy) is superior in predicting the absence of an offending lesion [17-19].
MAGNETIC RESONANCE IMAGINGMRI can be used to evaluate the nerve roots and surrounding anatomical structures with various imaging examinations. It is common and convenient to evaluate the intervertebral foramina by sagittal imaging using T1-weighted images and T2-weighted images. Wildermuth et al. [20] proposed a method for evaluating lumbar foraminal and canal stenoses using MRI. The author proposed a 4-stage scoring of lumbar foraminal stenosis using MRI and claimed that it can replace myelography. Lee et al. [21] proposed a new grading method that focused on the vertical or transverse direction of compression on MRI scans. Four grades have been developed for lumbar foraminal stenosis based on sagittal MRI findings. Grade 0 indicates no osteostenosis. Grade 1 refers to mild foramen stenosis with loss of perineural fat in 2 opposite directions, vertical or lateral. Grade 2 refers to moderate foraminal stenosis with 4-way perineural fat loss without nerve root morphological changes, both vertically and laterally. Grade 3 refers to severe foraminal stenosis indicating nerve root disruption or morphological changes. In total, 576 foramina from 96 patients were analyzed (L3–4 to L5–S1). In addition, they reported nearly perfect interobserver and intraobserver agreement (Figure 2) [21]. Other reports have indicated that the interobserver agreement for the Lee system was slightly higher and substantially correlated than that for the Wildermuth system. Although both systems are suitable for the assessment of lumbar foraminal stenosis, the Lee system showed slightly better interobserver agreement and clinical correlation, especially in younger patients [22].
Several reports have shown that examinations using coronal MRI are effective [23-25]. Since it is possible to compare the course of the nerve roots on both sides of the coronal section on MRI, foraminal stenosis can be diagnosed by confirming the difference in angle and the presence or absence of nerve swelling. In other words, foraminal stenosis is more likely to occur in nerves that swell or tilt than on the contralateral side.
In contrast, Takeuchi et al. [26,27] reported the usefulness of oblique coronal MRI compared to normal coronal MRI for diagnosing lumbar fifth nerve root entrapment. An oblique coronal MRI is a coronal image cut parallel to the line connecting the upper edge of the posterior wall of L4 and the lower edge of the anterior wall of L5 in the midsagittal section of the lumbar spine, just below the pedicle L5 nerve root and L4. Comparing the angle formed by the upper edge of the L5/S disc between the symptomatic and asymptomatic sides may lead to a more accurate diagnosis. It was reported that when the difference between the 2 foraminal spinal nerve angles is 10° or more, it can be detected with a sensitivity of 94% and a specificity of 91% (Figure 3).
MODERN SEQUENCES OF MRI1. 3D-MRI [8,28-33]Lateral spinal canal stenosis, which causes lumbar foraminal stenosis and FBSS, is difficult to detect using myelography. Moreover, parasagittal magnetic resonance (MR) has long been recommended for the investigation of lateral spinal regions and intraforaminal lesions. However, such MR images do not provide complete information and sometimes result in false-positive or false-negative results [8,33]. Yamada et al. [32] reported that 3D-MRI shows a higher detection rate of foraminal stenosis by visualizing the nerve root, including the intraforaminal part, considering the following suspicious findings (Figure 4): (1) transverse path of the nerve root and/or spinal nerve; (2) obscurity of the dorsal root ganglion; (3) spinal nerve indentation; and (4) nerve swelling. Byun et al. [30,31] focused on nerve root indentation and swelling and showed that 3D-MRI is effective for extraforaminal stenosis of the lumbosacral transitional vertebra. In addition, 3D-MRI originally based on T2-weighted images has been reported. However, considering the existence of intraforaminal perineural fat, 3D-MRI based on T1-weighted image was also verified and its performance was equivalent with imaging based on T2-weighted images [28].
2. Diffusion Tensor TractographyOikawa et al. [34] reported the usefulness of diffusion tensor tractography (DTT) in spinal canal stenosis visualization. Diffusion-weighted imaging (DWI) can provide information about the tissue microstructure by applying motion probe gradients in several directions to observe the random motion of water molecules confined within the tissue. DWI is widely used clinically to assess the central nervous system for the diagnosis of diseases such as acute stroke. Diffusion is described as “isotropic” if there is no rate of change of direction in the tissue. In contrast, water molecules tend to move along nerve fibers in neural tissue; this is called “anisotropic” diffusion. DTT uses diffusion tensor imaging (DTI) to visualize highly anisotropic nerve fiber tracts. Since DTT is based on DWI, it is expected to reflect the degree of nerve damage. Diffusion data can be used to determine quantitative diffusion values, such as the apparent diffusion coefficient and scalar fractional anisotropy (FA), which reflect the directionality of molecular diffusion. In the asymptomatic nerves, tractography clearly demonstrated all L3–S1 spinal nerve roots. Tractographic abnormalities were classified into 3 types according to their shape: "disrupt,” "narrowing,” and "tapering.” In addition to morphological abnormalities on tractography, the mean FA of symptomatic nerves was lower than that of the intact side. This study reported that tractography revealed nerve root abnormalities in lumbar degeneration and decreased FA in the symptomatic nerve roots (Figure 5). In contrast, Eguchi et al. [35] measured and reported the FA separately for the lumbar intraspinal zone, nerve root, and extraforaminal zone to distinguish between lumbar intraspinal and foraminal stenosis. A low FA value in the extraforaminal zone suggests foraminal stenosis. When the FA value and FA ratio cutoff values were established as 0.42% and 83.9%, respectively, the accuracy was high for the diagnosis of foraminal stenosis. This study showed that DTI parameters can be used to distinguish between lumbar intraspinal stenosis and foraminal stenosis [36,37].
CONCLUSIONLumbar foraminal stenosis is termed as a hidden zone and has been difficult to diagnose since its recognition. Even today, lumbar foraminal stenosis may be overlooked as a cause of sciatic pain and is considered one of the factors of FBSS. Even though an accurate diagnosis is difficult, it is essential. The evolution of noninvasive imaging examination by MRI has proven helpful for diagnosing lumbar foraminal stenosis. With 3D-MRI, oblique coronal MRI, and DTT, the depiction of nerve roots and surrounding structures has become clearer.
However, it is important for spine surgeons to correctly understand the mechanism and classification of lumbar degenerative stenotic diseases and to assess the examination results. Sometimes, it is important to use dynamic radiography, myelography, SNI, CT, and MRI techniques in combination to make a comprehensive diagnosis, considering its correspondence with the patient's symptoms.
Figure 1.Segmentation of the lateral part of the lumbar spinal canal and the compressed area of each pathology. Adapted from Kunogi. Spine Spinal Cord 2020;33:361-5 [11], with permission of copyright holder. 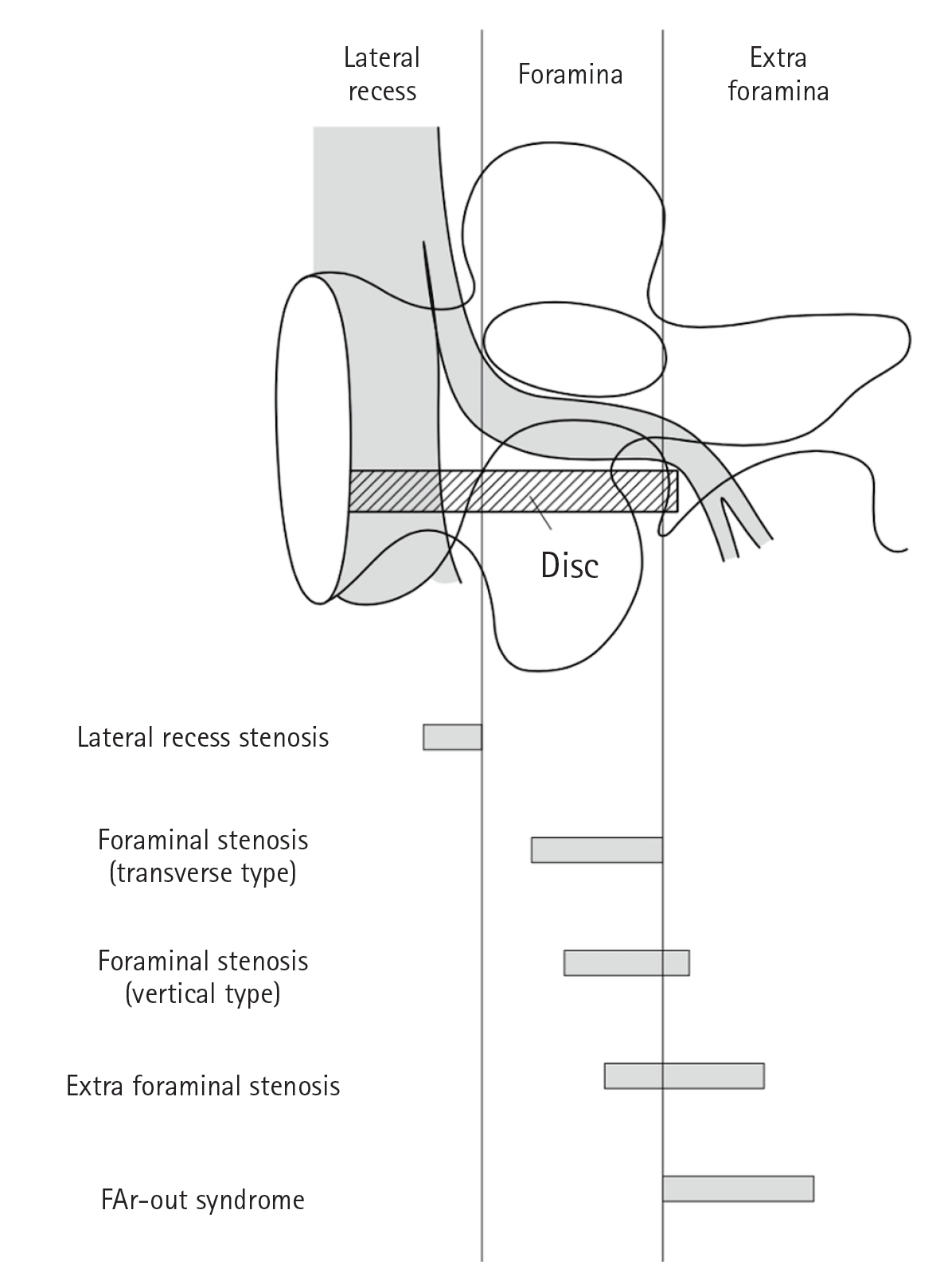
Figure 2.(A) Schematic illustrations of the 4-point-scale for grading foraminal stenosis on sagittal magnetic resonance imaging of the lumbar spine. Grade 0 (normal). A schematic diagram of the sagittal cross-section through the foramen shows the relationship between the foramen and surrounding structures. NR, nerve root; V, vertebral body; D, intervertebral disc; LF, ligamentum flavum; FJ, facet joint. (B) Grade 1 (mild foraminal stenosis). Schematic illustration showing perineural fat obliteration surrounding the nerve root in the transverse direction (arrows). Narrowing of the superior foraminal width was observed due to disc space narrowing and a thickened ligamentum flavum. No evidence of morphological changes was observed in the nerve roots. (C) Grade 1 (mild foraminal stenosis). Schematic illustration showing perineural fat obliteration surrounding the nerve root in the vertical direction (arrows). The foraminal height narrowed because of disc space narrowing and disco-osteophytic protrusion in the foraminal zone. No evidence of morphological changes was observed in the nerve roots. (D) Grade 2 (moderate foraminal stenosis). Schematic showing perineural fat obliteration surrounding the nerve root in 4 directions (vertical and transverse) (arrows) without morphological changes. Narrowing of the foraminal width and height due to disc space narrowing, a thickened ligamentum flavum, facet arthropathy, and disco-osteophytic protrusion in the foraminal zone. No evidence of morphological changes was observed in the nerve roots. (E) Grade 3 (severe foraminal stenosis). Schematic showing nerve root collapse or morphological changes (arrows) due to severe disc space narrowing, a severely thickened ligamentum flavum, facet arthropathy, and disco-osteophytic protrusion in the foraminal zone. Adapted from Lee et al. AJR Am J Roentgenol 2010;194:1095-8 [21], with permission of American Roentgen Ray Society. 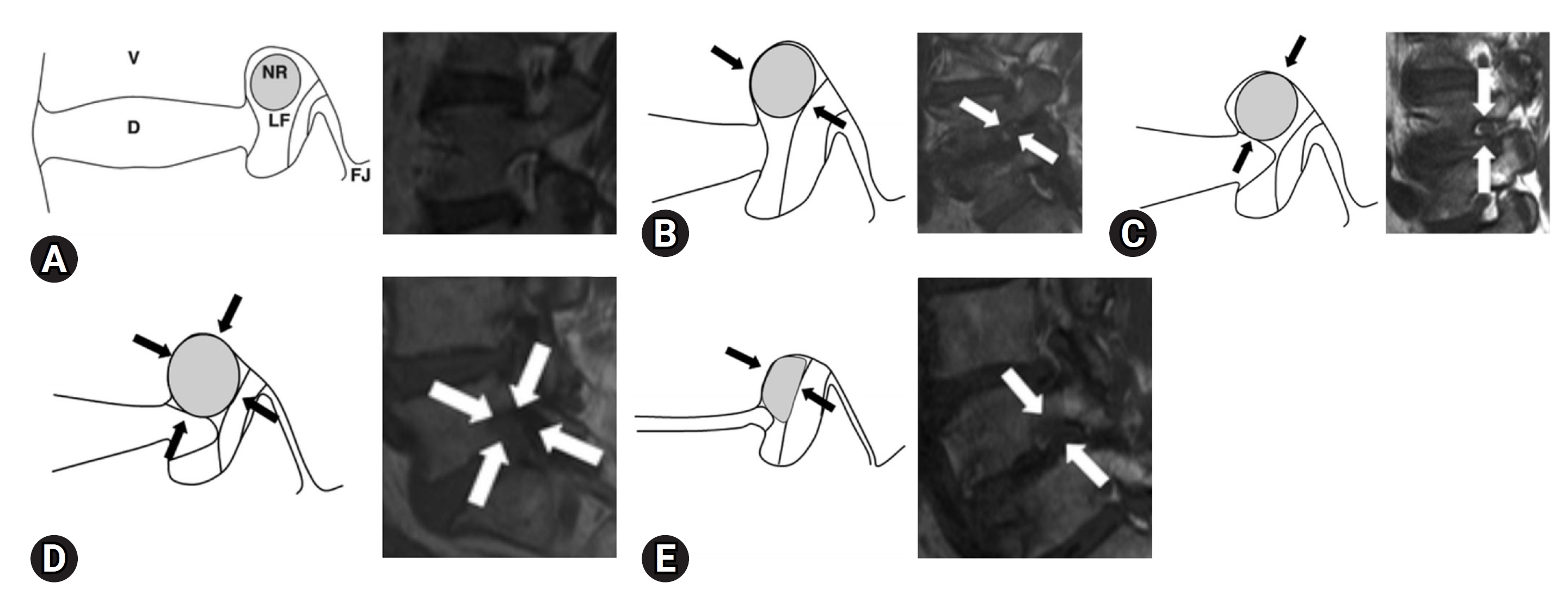
Figure 3.(A) Scout image in T2-weighted (sagittal section) oblique coronal magnetic resonance imaging. The white lines are parallel to the line passing the superior edge of the L4 posterior wall (arrowhead) and the inferior edge of the L5 anterior wall (blanked arrowhead). (B) Oblique coronal T2-weighted imaging. α, The angle between the right L5 spinal nerve (*) and the L5/S disc; β, The angle between the left L5 spinal nerve (▲) and the L5/S disc. ΔFSNA=α-β. FSNA, foraminal spinal nerve angle; P, pedicle. (C) (Case of foraminal stenosis): right L5 spinal nerve (asymptomatic side*), left L5 spinal nerve (symptomatic side A). (D) (Case of lateral spinal canal stenosis): right L5 spinal nerve (asymptomatic side*), left L5 spinal nerve (symptomatic side A) [26,27]. Adapted from Takeuchi et al. Spinal Surg 2016;30:93-4, with permission of copyright holder. 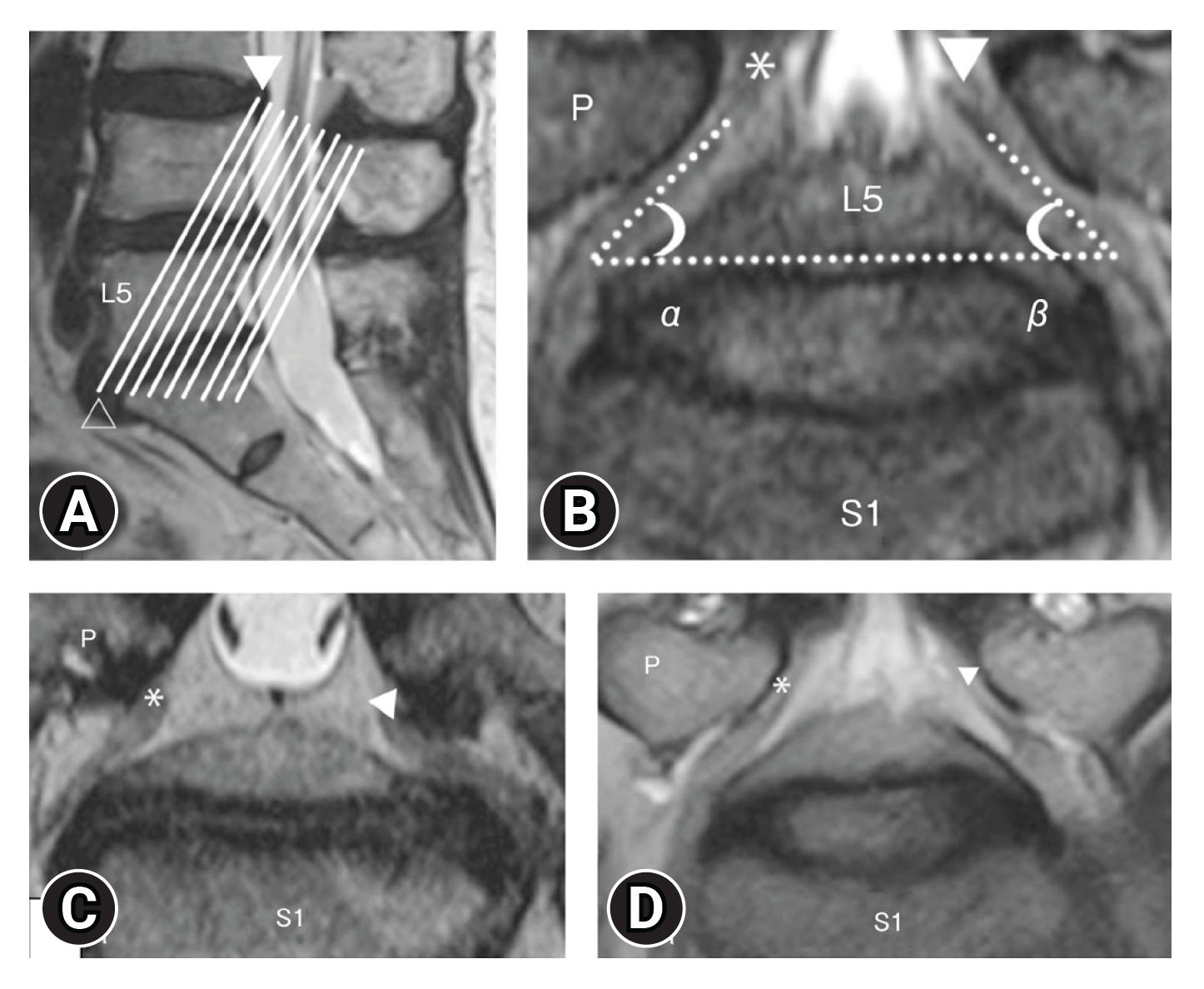
Figure 4.Highly suspicious findings of lumbar intra- and/or extraforaminal stenosis. (A) Transverse path of the nerve root and spinal nerve. (B) Obscurity of the spinal ganglion. (C) Constriction of the spinal nerve. (D) Nerve swelling. Adapted from Yamada et al. J Orthop Sci 2015;20:287-94 [32], with permission of Elsevier. 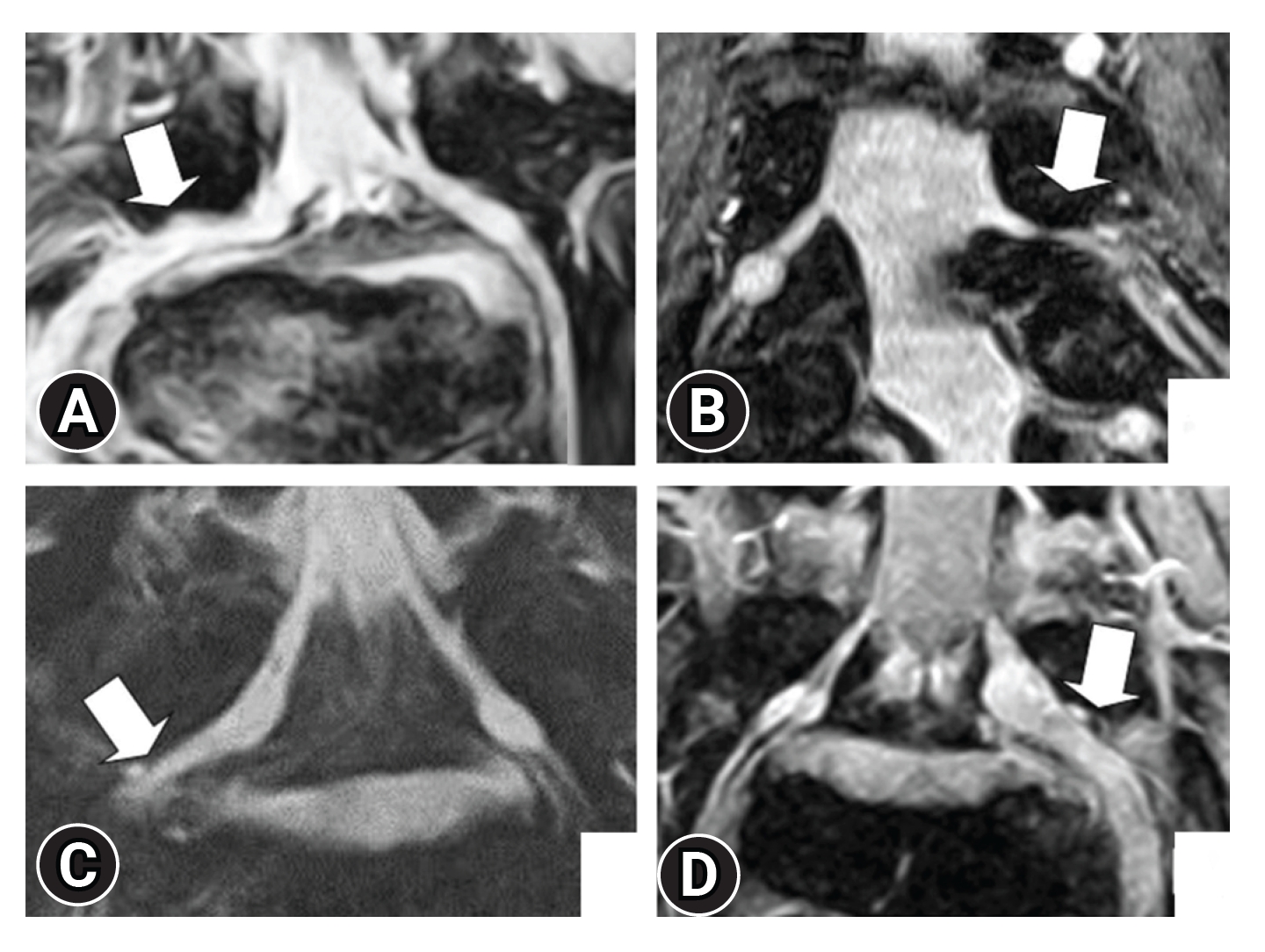
Figure 5.Abnormalities in tractography were classified into 3 types, disrupted, narrowing, and tapering, according to their shape (purple arrow). Tractography was defined as disrupted when it was not drawn continuously, narrowing when it continued unclearly compared to the normal side, and tapering when it was not drawn in the extraforaminal region. Adapted from Oikawa et al. Magn Reson Imaging 2015;33:956-61 [34], with permission of Elsevier. 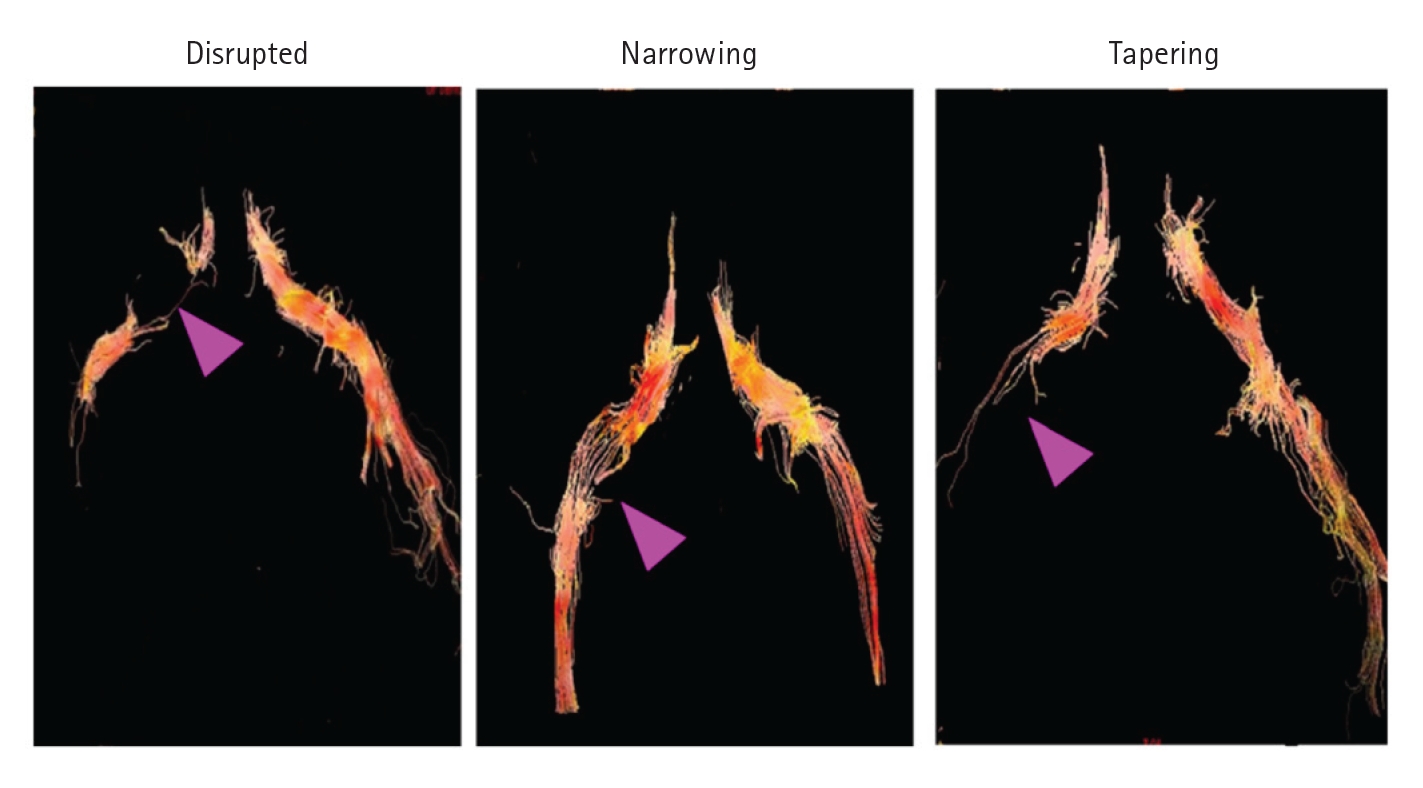
Table 1.Table 2.Multivariable predictors for the diagnosis of lumbar intra- and/or extraforaminal stenosis and the associated risk scoring system as a support tool [14] REFERENCES1. Gowers WR. A manual of diseases of the nervous system. 2nd ed. London: Churchill; 1981. p. 263–4.
2. Putti V. The Lady Jones lecture: on new conceptions in the pathogenesis of sciatic pain. Lancet 1927;2:53–60.
3. Danforth MS, Wilson PD. The anatomy of the lumbo-sacral region in relation to sciatic pain. J Bone Joint Surg 1925;23:109–60.
4. Briggs H, Krause J. The intervertebral foraminotomy for the relief of sciatic pain. J Bone Joint Surg 1945;27:475–8.
5. Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br 1954;36:230–7.
6. Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res 1976;115:4–5.
7. Macnab I. Negative disc exploration. An analysis of the causes of nerve‒root involvement in sixty‒eight patients. J Bone Joint Surg Am 1971;53:891–903.
8. Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine (Phila Pa 1976) 1991;16:1312–20.
9. Porter R, Hibbert C, Evans C. The natural history of root entrapment syndrome. Spine (Phila Pa 1976) 1984;9:418–21.
10. Nikaido T, Konno S. Concept and classification of lumbar spinal stenosis. Spine Spinal Cord 2020;33:342–6.
11. Kunogi J. Classification of the lumbar foraminal stenosis. Spine Spinal Cord 2020;33:361–5.
12. Yamada K, Aota Y, Higashi T, Ishida K, Nimura T, Konno T, et al. Lumbar foraminal stenosis causes leg pain at rest. Eur Spine J 2014;23:504–7.
13. Yamada H, Yoshida M, Minamide A, Nakagawa Y, Kawai M, Iwasaki H, et al. Clinical features of extraforaminal stenosis at lumbosacral junction. Rinsho Seikei Geka 2009;44:593–8.
14. Yamada H, Oka H, Iwasaki H, Endo T, Kioka M, Ishimoto Y, et al. Development of a support tool for the clinical diagnosis of symptomatic lumbar intra- and/or extra-foraminal stenosis. J Orthop Sci 2015;20:811–7.
16. Orita S, Inage K, Eguchi Y, Kubota G, Aoki Y, Nakamura J, et al. Lumbar foraminal stenosis, the hidden stenosis including at L5/S1. Eur J Orthop Surg Traumatol 2016;26:685–93.
17. Datta S, Everett CR, Trescot AM, Schultz DM, Adlaka R, Abdi S, et al. An updated systematic review of the diagnostic utility of selective nerve root blocks. Pain Physician 2007;10:113–28.
18. Sasso RC, Macadaeg K, Nordmann D, Smith M. Selective nerve root injections can predict surgical outcome for lumbar and cervical radiculopathy: comparison to magnetic resonance imaging. J Spinal Disord Tech 2005;18:471–8.
19. Wilson DJ, Allen G, Bullock S, Denton J. Lumbar nerve root blocks using MRI - the effectiveness and safety of ultrasound/MRI fusion image guidance. Br J Radiol 2022;95:20210599.
20. Wildermuth S, Zanetti M, Duewell S, Schmid MR, Romanowski B, Benini A, et al. Lumbar spine: quantitive and qualitative assessment of positional (upright flexion and extension) MR imaging and myelography. Radiology 1998;207:391–8.
21. Lee S, Lee JW, Yeom JS, Kim KJ, Kim HJ, Chung SK, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol 2010;194:1095–8.
22. Park HJ, Kim SS, Lee SY, Park NH, Rho MH, Hong HP, et al. Clinical correlation of a new MR imaging method for assessing lumbar foraminal stenosis. AJNR Am J Neuroradiol 2012;33:818–22.
23. Hashimoto M, Watanabe O, Hirano H. Extraforaminal stenosis in the lumbosacral spine. Efficacy of MR imaging in the coronal plane. Acta Radiol 1996;37:610–3.
24. Merter A, Shibayama M. Does "coronal root angle" serve as a parameter in the removal of ventral factors for foraminal stenosis at L5-S1 In stand-alone microendoscopic decompression? Spine (Phila Pa 1976) 2020;45:1676–84.
25. Park SH, Jeon I, Kim SW. Diagnostic values of ProSet magnetic resonance coronal source imaging for detecting symptomatic lesion in multiple lumbar foraminal stenosis. Clin Neurol Neurosurg 2016;150:185–9.
26. Takeuchi M, Wakao N, Kamiya M, Hirasawa A, Osuka K, Joko M, et al. Lumbar extraforaminal entrapment: performance characteristics of detecting the foraminal spinal angle using oblique coronal MRI. A multicenter study. Spine J 2015;15:895–900.
27. Takeuchi M, Ueda M, Aoyama M, Miyaoka K, Kawaguchi R, Niwa A, et al. Differential diagnosis using oblique coronal MRI of lumbar foraminal stenosis and canal stenosis. Spinal Surg 2016;30:93–4.
28. Hashimoto K, Tanaka Y, Tsubakino T, Hoshikawa T, Nakagawa T, Inawashiro T, et al. Imaging diagnosis of lumbar foraminal stenosis in the fifth lumbar nerve root: reliability and reproducibility of T1-weighted three-dimensional lumbar MRI. J Spine Surg 2021;7:502–9.
29. Aota Y, Niwa T, Yoshikawa K, Fujiwara A, Asada T, Saito T. Magnetic resonance imaging and magnetic resonance myelography in the presurgical diagnosis of lumbar foraminal stenosis. Spine (Phila Pa 1976) 2007;32:896–903.
30. Byun WM, Jang HW, Kim SW. Three-dimensional magnetic resonance rendering imaging of lumbosacral radiculography in the diagnosis of symptomatic extraforaminal disc herniation with or without foraminal extension. Spine (Phila Pa 1976) 2012;37:840–4.
31. Byun WM, Kim JW, Lee JK. Differentiation between symptomatic and asymptomatic extraforaminal stenosis in lumbosacral transitional vertebra: role of three-dimensional magnetic resonance lumbosacral radiculography. Korean J Radiol 2012;13:403–11.
32. Yamada H, Terada M, Iwasaki H, Endo T, Okada M, Nakao S, et al. Improved accuracy of diagnosis of lumbar intra and/or extra-foraminal stenosis by use of three-dimensional MR imaging: comparison with conventional MR imaging. J Orthop Sci 2015;20:287–94.
33. Cramer GD, Cantu JA, Dorsett RD, Greenstein JS, McGregor M, Howe JE, et al. Dimension of the lumbar intervertebral foramina as determined from the sagittal plane magnetic resonance imaging scans of 95 normal subjects. J Manipulative Physiol Ther 2003;26:160–70.
34. Oikawa Y, Eguchi Y, Inoue G, Yamauchi K, Orita S, Kamoda H, et al. Diffusion tensor imaging of lumbar spinal nerve in subjects with degenerative lumbar disorders. Magn Reson Imaging 2015;33:956–61.
35. Eguchi Y, Ohtori S, Suzuki M, Oikawa Y, Yamanaka H, Tamai H, et al. Discrimination between lumbar intraspinal stenosis and foraminal stenosis using diffusion tensor imaging parameters: preliminary results. Asian Spine J 2016;10:327–34.
|
|
|||||||||||||||||||||||||||||||||||||||||||