AbstractObjectiveTo describe the minimally invasive, microscopic-assisted over-the-top technique and report its clinical and radiological outcomes in cases of spinal canal stenosis and first-degree degenerative lumbar spondylolisthesis.
MethodsTwenty-two patients with grade I degenerative spondylolisthesis and spinal canal stenosis who underwent microscopic decompression without fusion between April 2017 and December 2020 were included in the study.
ResultsThe study population included 13 men and 9 women, with an average age of 66.7 years (range, 55–79 years) and a mean duration of symptoms of 14.8±11.6 months. The mean follow-up was 49.3 months (range, 24–67 months). At the last follow-up, 13 patients were fully satisfied, 7 patients were partially satisfied, and 2 patients (9%) were not satisfied and required revision surgery with fusion. At the final follow-up, the mean leg pain numerical pain rating scale (NPRS), back pain NPRS, Oswestry Disability Index score, and Japanese Orthopaedic Association Back Pain Evaluation Questionnaire showed significant improvements in all patients, and no patients showed progression of the degree of spondylolisthesis.
INTRODUCTIONDegenerative spondylolisthesis (DS) is an acquired anterior displacement of one vertebra over the subjacent vertebra in the sagittal plane, associated with degenerative changes such as facet arthropathy, ligamentous malfunctions or disc degeneration, without an associated disruption or defect in the vertebral ring [1-4]. Symptoms from DS range from none to, occasional low back pain (LBP) to incapacitating mechanical back pain associated with radiculopathy from nerve root compression and/or neurogenic claudication [5]. Surgical treatment in indicated once conservative measures fail [6,7].
DS most commonly affects the L4–5 level [8] due to the anatomy of the facet joints and biomechanical load distribution across the L4–5 segment. Grades I and II slips as per the Myerding classification are more common than high grade slips (III and above) [9,10]. In most cases, activity modification, analgesics and intermittent bracing are sufficient to control symptoms [1,2,11]. However, it has been estimated that 10% to 15% of patients seeking treatment will eventually have surgery [11].
Currently, there is widespread variation in the surgical modalities used to manage this heterogeneous condition, with factors such as patient age, medical comorbidities, occupation, clinical symptoms [11], imaging findings of ‘dynamic instability’ [12] and surgeon preference all influencing the management strategy [11,12]. Surgical interventions can be broadly classified into decompression alone or decompression with fusion (posterolateral fusion/interbody fusion) when obvious dynamic instability is present [12,13]. Although the conventional open techniques of decompression (which involve laminectomy) remain the gold standard of treatment [12,14], problems with paraspinal musculature denervation [15] and possibility of secondary lumbar instability [16] and creation of dead space [17] resulted in increased interest in less invasive techniques [18-22], with reported noninferior clinical outcomes [21,22]. Among them, the minimally invasive surgical (MIS) decompression via a unilateral laminotomy with bilateral decompression—the ‘over-the-top’ approach, is thought to be less destabilizing than all other techniques [23-26].
MIS with unilateral laminotomy has been associated with higher patients’ satisfaction, reduced likelihood of slip progression, and reduced reoperation and secondary fusion [21] compared to standard open techniques of decompression. Even in cases that required fusion, minimally invasive fusion was found to have less hospital stay, less intraoperative blood less and rates of transfusions [27,28], improved muscle bulk [29], less postoperative narcotic use [30] along with comparable functional outcomes [27,28,30].
While several innovations and techniques exist to perform less invasive decompressions, including subarticular fenestration and multiple laminotomies [19-21], microscopic decompression [25,31] and tubular decompression [32-35], the aim of this study is to report our outcomes of microscopic-assisted decompression in first-degree symptomatic DS cases through over-the-top technique, particularly regarding clinical improvement and radiological progression of instability. Despite the presence of similar published reports regarding the same technique [24-26], the existing prospective studies were few and the results were nonconsistent specially regarding slip progression. We aim to present a prospective case series performed by the same surgeons, which could add to the available evidence regarding this approach, highlight the specific indications of the procedure; and revisit the surgical technique, which can affect the clinical and radiographic outcomes.
MATERIALS AND METHODSAfter obtaining an Institutional Review Board (IRB) approval, 22 consecutive patients, between April 2017 and December 2020 with spinal canal stenosis associated with first-degree DS were included in this prospective study
1. Inclusion Criteria- Patients with spinal canal stenosis
- Presence DS (grade I)
- Radiculopathy or neurogenic claudication are the main complaint (no or mild LBP)
- Failed conservative treatment for at least 3 months
All the patients received conservative treatment primarily, in the form of analgesics, intermittent bracing and therapeutic physical therapy program (postural instruction, lumbopelvic mobilization exercises, and a flexion-based exercises) [36]. Five patients received additional epidural steroid injection with no improvement.
2. Exclusion Criteria- Patients grade II and above spondylolisthesis
- Patients with unstable spondylolisthesis in radiographs. Unstable spondylolisthesis was defined as gross segmental motion or anteroposterior translation on static end-range flexion and extension lateral radiographs of greater than 2 mm [37-39].
- Patients with any associated lytic spondylolisthesis (isthmic spondylolisthesis)
- Patients with considerable LBP (moderate to severe) according to numerical pain rating scale (NPRS) for LBP (above 6)
- Patients with foraminal stenosis
Patients who met the inclusion criteria were evaluated radiologically through standard anteroposterior, lateral neutral, flexion and extension plain radiographs as well as lumbosacral magnetic resonance imaging. A thorough physical exam was performed, and neuromuscular sensory and motor evaluation was completed. Outcomes data including the NPRS scale [40] for back and leg pain (1–10) and the Oswestry Disability Index (ODI) and the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ) [41] scoring system were collected.
3. Surgical TechniqueSurgical technique has been performed based on the original description of the approach [23-25]. After obtaining a written informed consent, patients were brought to the operating room and general anaesthesia was induced. Patients were then transferred to the operating table in a prone position. Level was identified by C-arm imaging and a midline 2-cm incision was made. The lumbodorsal fascia was unilaterally opened on the more symptomatic side and dissection was carried down subperiosteally to the intended surgical level. Laminae of the adjacent vertebrae were exposed and the interlaminar window was cleaned until the yellow ligament (ligamentum flavum) is identified. Self-retaining retractors were placed and standard ipsilateral laminotomy was performed under microscopic magnification. The scope is then angulated, and the bed tilted contralaterally to the deepest portion of interspinous ligament to allow the posterior surface of the contralateral ligamentum flavum to be seen. A probe is used to confirm that the anterior surface of the ligamentum is free from adhesion to the dura and the ligamentum is then resected from above downwards and medial to lateral. After confirming that exiting and traversing roots are well decompressed on both sides the wound is closed in layers and subcutaneous tissues are injected with a long-acting local anaesthetic to reduce incisional pain. Adjacent stenotic levels if present were addressed similarly.
Early return to ambulation and normal activities of daily living is encouraged. Postoperative rehabilitation was performed by a formal physiotherapy program that begins core muscle stabilization and aerobic activities after 1–2 weeks. Patients were followed at week 2, 6, and 12 postoperatively and then scheduled for follow-up biannually. Outcomes data collected included NPRS for back and leg pain, JOABPEQ, ODI. At the final follow-up, all patients were asked if they were satisfied after doing the surgery with 3 options for answers either; fully satisfied, partially satisfied, or not satisfied at all. Radiological follow-up was done with dynamic radiographs on biannual basis. Analgesics were prescribed for 2-4 weeks after surgery.
RESULTSPatients were 13 males and 9 females with an average age of 66.7 years (range, 55–79 years). Mean duration of symptoms was 14.8±11.6 months. Surgery was done on 2 levels in 5 cases, where stenosis was present significantly in 2 levels, and discectomy was done on 2 cases where significant disc prolapse was present (Figure 1). Spondylolisthesis was present in L4/5 level in 20 cases, and 2 cases were involving L3/4 level. The average operation time was 55±7.95 minutes, average blood loss was 72±21.96 mL, and average hospital stay was 1.9±0.57 days.
No intraoperative complications were detected. The mean follow-up was 49.3 months (range, 24–67 months). At the last follow-up, 13 patients reported to be fully satisfied, 7 patients reported to be partially satisfied while 2 patients (9%) were not satisfied at all and all required revision formal posterior decompression and transforaminal lumbar interbody fusion (TLIF) due to nonresolution of leg pain and claudication. At the final follow-up, the mean lower limb pain NPRS scores changed from 8.35±1.19 preoperatively to 1.6±1.1 (p<0.00001) while the mean LBP NPRS scores changed from 3.4±1.7 preoperatively to 0.95±0.92 at the final follow-up (p<0.00001) (Figure 2). The mean preoperative ODI score was 66.8%±8.3%, decreasing to 25%±4.9% at the final follow-up (p<0.00001). Regarding the JOABPEQ, significant improvement in all ODI components was noticed at 1-year follow-up (Table 1).
DISCUSSIONThis prospective study investigates the clinical and radiological outcomes of minimally invasive microscopic-assisted decompression surgery in management of spinal canal stenosis associated with grade 1 DS cases. After a mean follow-up of 49.3 months, good to excellent outcomes have been achieved in 20 cases (91%) while 2 cases (9%) required revision with TLIF.
Most cases of spinal stenosis associated with first-degree DS are managed conservatively with good results [1,2]. For those who require surgery, decompression and instrumented fusion has been one of the most chosen surgical approaches. Fusion surgery has its own demerits including but not limited to longer recovery, potential for complication from hardware placement, pseudoarthrosis or adjacent segment degeneration, to name a few [22,42].
To avoid the issues related to fusion surgery, various surgical techniques for decompression surgery, without fusion have been described in cases of symptomatic canal stenosis and stable spondylolisthesis, with overall satisfactory clinical results [24-26], yet slip progression after surgery, and hence the need for revision surgery remains a potential drawback after a nonfusion surgery for spondylolisthesis [43,44]. With improvement of surgical instruments and techniques, the use of surgical microscopy allows for utilization of smaller incision, and less soft tissue and bony violation during the decompression surgery [45], which may result in a "more stable" decompression that could potentially decrease the incidence of postoperative instability and revision surgery.
Several studies have reported about the clinical outcomes of microscopic, assisted decompression in low grade spondylolisthesis [23-26,44-48]. Following this technique, some reported no significant slip progression in short to midterm follow-up [24,26,47], while others reported significant slip progression [44,45]. Minamide et al. [44] reported significant clinical improvement while slip progression occurred in 19 of 242 cases (7.9%). Jang et al. [46], reported a slip progression in 7 of 21 cases (33%). Nakanishi et al. [26] reported mild increased mean slip angle postoperatively in static but not in dynamic views. They reported that overall slip progression was insignificant. As for reoperation rate, Müslüman et al [24] reported that one case (1.2%) from his 84 cases series required fusion surgery.
Compared to microscopic decompression, formal open decompression without fusion was reported to cause postoperative instability incidence reaching 26%, as reported by Inose et al. [49]. Interestingly, they reported the same percent of slip progression among decompression plus stabilization group (without fusion). In a metanalysis published by Scholler et al [21], minimally invasive decompression was found to result in lower reoperation and fusion rates, less slip progression, and greater patient satisfaction than open surgery.
Our clinical results were comparable to the above reported studies- in terms of clinical improvement, while none of our cases showed significant slip progression. Two of our cases required formal decompression and fusion due to worsening of leg pain and LBP. These 2 cases were one of our first few cases, which certainly can be attributed to surgical technique as we progressed along the learning curve. Our study shows much better results (blood loss, operative time, and hospital stay) in comparison to fusion techniques, as would be expected with decompression only surgery [13,14,16].
We have several limitations in our study; the limited number of patients, short period of follow-up, and absence of control group. A comparative randomized study with longer term follow-up and larger number of cases would be ideal to assess the success of the technique, especially in regarding the occurrence of instability and the need for revision.
CONCLUSIONWe believe microscopic decompression using "over-the-top technique" is a viable option for patients with grade I DS and spinal stenosis with predominant leg pain symptoms. It allows for smaller incision, shorter hospital stay, minimal wound complications and overall good clinical outcomes without increasing the risk of instability. Future research should focus on long term outcomes of this technique.
Figure 1.Preoperative PXRs (A, B) and MRI (C, D) of a 64-year-old female with first-degree degenerative spondylolisthesis and symptoms of lumbar canal stenosis. Decompression was performed in 2 levels (L3–4 and L4–5) with excision of the far lateral disc protrusion. (E, F) Follow-up PXRs 26 months postoperatively, showing no progression of instability on dynamic views. PXR, plain x-ray; MRI, magnetic resonance imaging. 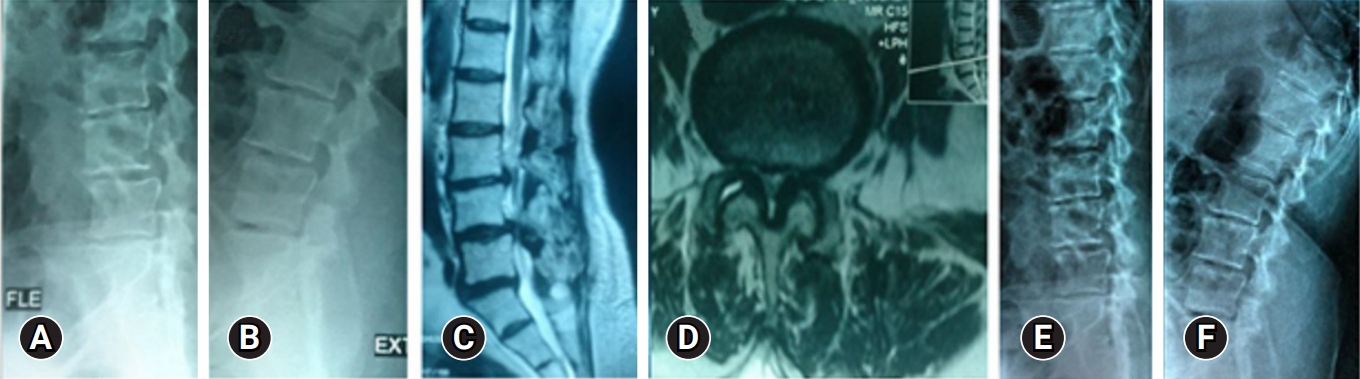
Figure 2.Postoperative improvements in lower limb pain (LLP) and low back pain (LBP) numerical pain rating scale (NPRS) scores. 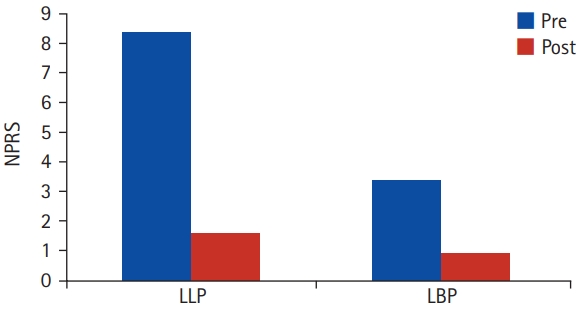
Figure 3.Preoperative PXRs (A, B) and MRI (C, D) of a 55-year-old man with first-degree L4–5 degenerative spondylolisthesis and symptoms of lumbar canal stenosis. Decompression was performed at one level (L3–4 and L4–5) with excision of the far lateral disc protrusion. (E, F) Follow-up PXRs 35 months postoperatively, showing no progression of instability on dynamic views. PXR, plain x-ray; MRI, magnetic resonance imaging. 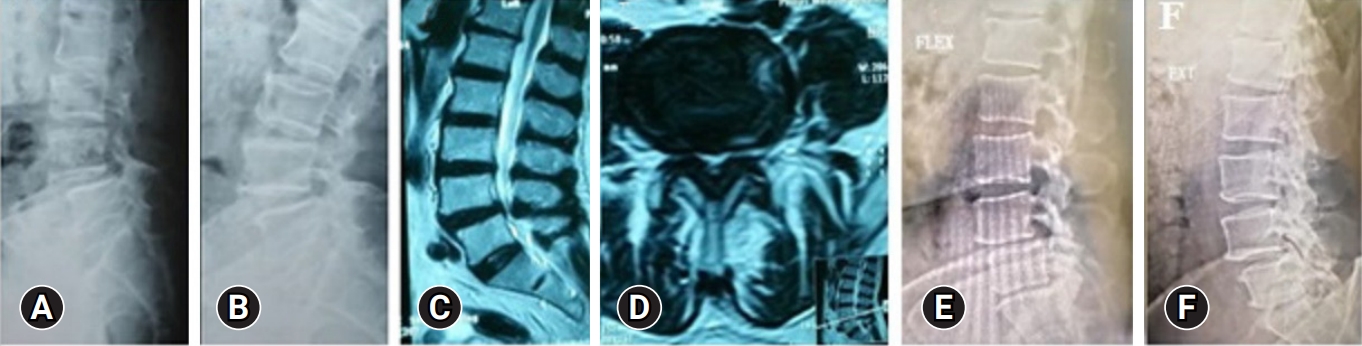
Table 1.Japanese Orthopaedic Association Back Pain Evaluation Questionnaire scores REFERENCES1. Matz PG, Meagher RJ, Lamer T, Tontz WL Jr, Annaswamy TM, Cassidy RC, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J 2016;16:439–48.
2. Atalay B, Gadjradj PS, Sommer FS, Wright D, Rawanduzy C, Ghogawala Z, et al. Natural history of degenerative spondylolisthesis: a systematic review and meta-analysis. World Neurosurg 2023 Jun 2:S1878-8750(23)00758-1. doi: 10.1016/j.wneu.2023.05.112. [Epub].
3. Wiltse LL, Winter RB. Terminology and measurement of spondylolisthesis. J Bone Joint Surg Am 1983;65:768–72.
4. Lan Z, Yan J, Yang Y, Xu Q, Jin Q. A review of the main classifications of lumbar spondylolisthesis. World Neurosurg 2023;171:94–102.
5. Gagnet P, Kern K, Andrews K, Elgafy H, Ebraheim N. Spondylolysis and spondylolisthesis: a review of the literature. J Orthop 2018;15:404–7.
6. Samuel AM, Moore HG, Cunningham ME. Treatment for degenerative lumbar spondylolisthesis: current concepts and new evidence. Curr Rev Musculoskelet Med 2017;10:521–9.
7. Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 2007;356:2257–70.
8. Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: a systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat 2016;11:39–52.
9. Meyerding HW. Spondylolisthesis; surgical fusion of lumbosacral portion of spinal column and interarticular facets; use of autogenous bone grafts for relief of disabling backache. J Int Coll Surg 1956;26(5 Part 1):566–91.
10. Koslosky E, Gendelberg D. Classification in brief: the Meyerding Classification System of spondylolisthesis. Clin Orthop Relat Res 2020;478:1125–30.
11. García-Ramos CL, Valenzuela-González J, Baeza-Álvarez VB, Rosales-Olivarez LM, Alpizar-Aguirre A, Reyes-Sánchez A. Degenerative spondylolisthesis I: general principles. Acta Ortop Mex 2020;34:324–8.
12. Schroeder GD, Kepler CK, Kurd MF, Vaccaro AR, Hsu WK, Patel AA, et al. Rationale for the surgical treatment of lumbar degenerative spondylolisthesis. Spine (Phila Pa 1976) 2015;40:E1161–6.
13. Duculan R, Fong AM, Cammisa FP, Sama AA, Hughes AP, Lebl DR, et al. High preoperative expectations and postoperative fulfillment of expectations two years after decompression alone and decompression plus fusion for lumbar degenerative spondylolisthesis. Spine J 2023;23:665–74.
14. Gatam AR, Gatam L, Phedy, Mahadhipta H, Ajiantoro, Aprilya D. Full endoscopic lumbar stenosis decompression: a future gold standard in managing degenerative lumbar canal stenosis. Int J Spine Surg 2022;16:821–30.
15. Sihvonen T, Herno A, Paljärvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine (Phila Pa 1976) 1993;18:575–81.
16. Johnsson KE, Redlund-Johnell I, Udén A, Willner S. Preoperative and postoperative instability in lumbar spinal stenosis. Spine (Phila Pa 1976) 1989;14:591–3.
17. Tuite GF, Stern JD, Doran SE, Papadopoulos SM, McGillicuddy JE, Oyedijo DI, et al. Outcome after laminectomy for lumbar spinal stenosis. Part I: clinical correlations. J Neurosurg 1994;81:699–706.
18. Kim HS, Wu PH, Jang IT. Rationale of endoscopic spine surgery: a new paradigm shift in spine surgery from patient’s benefits to public interest in this new era of pandemic. J Minim Invasive Spine Surg Tech 2021;6(Suppl 1):S77–80.
19. Papavero L, Schawjinski K, Ali N, Oehm J, Pietrek M, Schoeller K. Lumbar spinal stenosis: ipsilateral facet-sparing unilateral laminotomy for bilateral decompression: technical note and preliminary results. J Minim Invasive Spine Surg Tech 2021;6:147–55.
20. Van Isseldyk F, Liu Y, Lim WJ, Kim YJ, Kim JH, Ryu KS, et al. Full-endoscopic foraminotomy in degenerative spondylolisthesis: a “module-based” approach for surgical planning and execution. J Minim Invasive Spine Surg Tech 2022;7:28–36.
21. Schöller K, Alimi M, Cong GT, Christos P, Härtl R. Lumbar spinal stenosis associated with degenerative lumbar spondylolisthesis: a systematic review and meta-analysis of secondary fusion rates following open vs minimally invasive decompression. Neurosurgery 2017;80:355–67.
22. Austevoll IM, Hermansen E, Fagerland MW, Storheim K, Brox JI, Solberg T, et al. Decompression with or without fusion in degenerative lumbar spondylolisthesis. N Engl J Med 2021;385:526–38.
23. Kim HS, Wu PH, Jang IT. Clinical results and review of techniques of lumbar endoscopic unilateral laminotomy with bilateral decompression (LE-ULBD) for lumbar stenosis. J Minim Invasive Spine Surg Tech 2021;6(Suppl 1):S117–22.
24. Müslüman AM, Cansever T, Yılmaz A, Çavuşoğlu H, Yüce İ, Aydın Y. Midterm outcome after a microsurgical unilateral approach for bilateral decompression of lumbar degenerative spondylolisthesis. J Neurosurg Spine 2012;16:68–76.
25. Dohzono S, Matsumura A, Terai H, Toyoda H, Suzuki A, Nakamura H. Radiographic evaluation of postoperative bone regrowth after microscopic bilateral decompression via a unilateral approach for degenerative lumbar spondylolisthesis. J Neurosurg Spine 2013;18:472–8.
26. Nakanishi K, Tanaka N, Fujimoto Y, Okuda T, Kamei N, Nakamae T, et al. Medium-term clinical results of microsurgical lumbar flavectomy that preserves facet joints in cases of lumbar degenerative spondylolisthesis: comparison of bilateral laminotomy with bilateral decompression by a unilateral approach. J Spinal Disord Tech 2013;26:351–8.
27. Villavicencio AT, Burneikiene S, Roeca CM, Nelson EL, Mason A. Minimally invasive versus open transforaminal lumbar interbody fusion. Surg Neurol Int 2010;1:12.
28. Wong AP, Smith ZA, Stadler JA 3rd, Hu XY, Yan JZ, Li XF, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF): surgical technique, long-term 4-year prospective outcomes, and complications compared with an open TLIF cohort. Neurosurg Clin N Am 2014;25:279–304.
29. Wu PH, Kim HS, An JW, Kim M, Lee I, Park JS, et al. Prospective cohort study with a 2-year follow-up of clinical results, fusion rate, and muscle bulk for uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion. Asian Spine J 2023;17:373–81.
30. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech 2011;24:479–84.
31. Krishnan A, Kim HS, Raj A, Dave BR. Expanded indications of full endoscopic spine sugery. J Minim Invasive Spine Surg Tech 2021;6(Suppl 1):S130–56.
32. Kulkarni AG, Khandge AKV, Yeshwanth T. Single incision tubular decompression to treat multi-level lumbar spinal stenosis: a retrospective review. J Minim Invasive Spine Surg Tech 2022;7:46–32.
33. Kim S, Park GJ, Lee JU, Kim KH, Kim DH. Comparative study of the outcomes of unilateral biportal endoscopic discectomy and tubular microdiscectomy based on the visual analogue scale, Oswestry Disability Index, and Short-form 36. J Minim Invasive Spine Surg Tech 2022;7:243–50.
34. Mehta RP, Patel AS, Jain S, Ruparel S, Kire N, Upadhyaya M, et al. Learning curve of MIS-TLIF using 22 mm-tubular retractor in degenerative spondylolisthesis (grade 1-2) - a review over 100 cases. J Minim Invasive Spine Surg Tech 2020;5:20–5.
35. Kulkarni AG, Yeshwanth T. Full endoscopic spine surgery in the view of tubular endoscopic spine surgery. J Minim Invasive Spine Surg Tech 2021;6(Suppl 1):S97–102.
36. Simotas AC, Dorey FJ, Hansraj KK, Cammisa F Jr. Nonoperative treatment for lumbar spinal stenosis. Clinical and outcome results and a 3-year survivorship analysis. Spine (Phila Pa 1976) 2000;25:197–203.
37. Hiratsuka S, Takahata M, Hojo Y, Kajino T, Hisada Y, Iwata A, et al. Increased risk of symptomatic progression of instability following decompression for lumbar canal stenosis in patients receiving chronic glucocorticoids therapy. J Orthop Sci 2019;24:14–8.
38. Spina N, Schoutens C, Martin BI, Brodke DS, Lawrence B, Spiker WR. Defining Instability in degenerative spondylolisthesis: surgeon views. Clin Spine Surg 2019;32:E434–9.
39. Lee CY, Park BM, Kim TW, Lee SH. Clinical implication of mid-range dynamic instability in lumbar degenerative spondylolisthesis. Asian Spine J 2020;14:507–12.
40. Suzuki H, Aono S, Inoue S, Imajo Y, Nishida N, Funaba M, et al. Clinically significant changes in pain along the Pain Intensity Numerical Rating Scale in patients with chronic low back pain. PLoS One 2020;15:e0229228.
41. Fukui M, Chiba K, Kawakami M, Kikuchi S, Konno S, Miyamoto M, et al. JOA back pain evaluation questionnaire: initial report. J Orthop Sci 2007;12:443–50.
42. Cheung JP, Cheung PW, Cheung KM, Luk KD. Decompression without fusion for low-grade degenerative spondylolisthesis. Asian Spine J 2016;10:75–84.
43. Epstein NE. Decompression in the surgical management of degenerative spondylolisthesis: advantages of a conservative approach in 290 patients. J Spinal Disord 1998;11:116–22.
44. Minamide A, Yoshida M, Simpson AK, Nakagawa Y, Iwasaki H, Tsutsui S, et al. Minimally invasive spinal decompression for degenerative lumbar spondylolisthesis and stenosis maintains stability and may avoid the need for fusion. Bone Joint J 2018;100-B:499–506.
45. Lee JH, Choi KC, Shim HK, Shin SH, Lee DC. Percutaneous biportal endoscopic surgery for lumbar degenerative diseases. J Minim Invasive Spine Surg Tech 2017;2:15–9.
46. Jang JW, Park JH, Hyun SJ, Rhim SC. Clinical outcomes and radiologic changes after microsurgical bilateral decompression by a unilateral approach in patients with lumbar spinal stenosis and grade i degenerative spondylolisthesis with a minimum 3-year follow-up. Clin Spine Surg 2016;29:268–71.
47. Lee YS, Choi JC, Oh SH, Park SR, Park SJ, Cho NI. Semi-circumferential decompression: microsurgical total en-bloc ligamentum flavectomy to treat lumbar spinal stenosis with grade I degenerative spondylolisthesis. Clin Orthop Surg 2015;7:470–5.
|
|
|||||||||||||||||||||||||||||||||||||||