AbstractObjectiveTo describe the presentation spectrum of postoperative spondylodiscitis (POSe) following transforaminal endoscopic lumbar discectomy and to report the outcomes of transforaminal lumbar interbody fusion (TLIF).
MethodsThis study analyzed all patients with the classic features of POSe who underwent index surgery elsewhere and presented to us. They had not responded to conservative care for 3 weeks and were operated further with open TLIF. The treatment response was judged by the declining values of inflammatory markers, improvements in mobility, and decreases in pain. Patients' outcomes were analyzed using a visual analogue scale (VAS), the Oswestry Disability Index (ODI), and the occurrence of complications. Radiological outcomes were assessed by fusion and implant stability. The spectrum of the demographic presentation was analyzed. PubMed was searched to find the incidence of POSe and the spectrum of organisms involved.
ResultsFifteen patients were operated primarily by interventionalists and four by surgeons among 19 POSe patients who finally underwent TLIF at Stavya Spine Hospital & Research Institute. Organism culture positivity was found in 10 and no culture results were present in 9 cases. All TLIF cases had a follow-up of 52.94±13.66 months (range, 28–71 months). The preoperative back pain VAS improved from 9.47±0.61 (8–10) to 0.42±0.50 (0–1). The leg pain VAS improved from 5.78±4.19 (6–10) to 0.52±0.61 (0–1). The preoperative ODI improved from 87.01±7.70 (73.33–97.79) to 7.36±8.14 (0–26.67). No major complications occurred. Cure of infection and stable reconstruction with fusion were achieved in all patients.
INTRODUCTIONTransforaminal endoscopy lumbar surgery (TELD) is a full endoscopic spine surgery (FESS) and is challenging standard of care for lumbar disc herniation (LDH) and other advanced indications. It has distinct advantages and fewer complication rates as compared to other techniques [1-5]. The benefits of TELD are related to local anesthesia and negligible manipulation of compromised neural tissues. Less trauma, reduced bleeding, quicker pain reduction, negligible scar, faster job resumption, day care surgery, rapid recovery, and decreased morbidity [6,7]. Immediate surgery if required without lengthy preanesthetic preparations can be done in cauda equina syndrome and elderly patients with comorbidities [8-11]. Though, the now-evolved epidural target and objectified decompression and outcomes brings with it a longer learning curve for spine surgical practitioners [12]. Postoperative spondylodiscitis (POS) is a dreaded infective complication which can occur after open (OD) or micro lumbar discectomy (MLD), leading to disabling pain, instability, with or without serious neurological affection needing protracted conservative treatment. Nonresponsive cases mandate operative treatment. Though different treatments have been discussed and reported extensively, unpredictability has not been solved [13,14]. POS following transforaminal endoscopic lumbar discectomy (POSe) is also reported. Increased TELD procedures also has led to the simultaneous rise of POSe complications [15-17]. It responds to conservative approach if recognized early. But if a phase where mechanical instability ensues with destruction then it will lead to delayed conservative recovery and may necessitate fusion surgery for better outcome [18-22]. Transforaminal lumbar interbody fusion (TLIF) in POS has good outcomes in the reported literature [22-24]. This study is primarily a retrospective study to report the clinical efficacy of TLIF in POSe. Secondarily, it was undertaken to report the presentation spectrum of POSe.
MATERIALS AND METHODSThis retrospective study was approved by the Stavya Spine Hospital & Research Institute Institutional Ethic Committee and registered on CTRI (Clinical Trial Registry India/2019/08/020560). From April 2014 to 2019 a total of 19 patients received treatment at our institute for POSe. Patients who were operated elsewhere by TELD and had presented to us with POSe and were then operated by us for TLIF were included. All had symptoms of fever and back pain with or without leg pain or motor deficit. Confirmed clinicoradiologically (radiographs/magnetic resonance imaging [MRI]) diagnosed POSe, not responding to conservative management within 3 weeks were operated by TLIF. Informed consent was obtained from all the patients before surgery. Identified patients were reviewed for demographics that included age, sex, body mass index, days of symptom before the index surgery, onset of symptoms of POSe following index surgery (days), VAS back pain, VAS leg pain, and neurologic symptoms. Motor weakness was recorded using the Medical Research Council grading scale from 0 to 5. A score of less than 3 was considered as motor weakness and greater than 3 was recovered. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) tests reports were noted. The operator, whether an index orthopedic/neurosurgeon or interventionist (interventional radiologist/pain practitioner/interventional physician/anesthesiologist) was also noted and analyzed. All patients were operated for TLIF under general anesthesia in a prone position and with midline exposure. Locally harvested posterior spinal element bone grafts and an interbody titanium banana cage were used in the TLIF procedure. In the case of the suspected aggressive organism, a tricortical facet bone graft was used as strut support instead of a cage. Free hand pedicle screws were inserted. The tissue/pus removed was sent for microbiological culture and sensitivity along with histopathological examination. Sterile preservative formalinised container for histopathological tissue and BACTEC culture system bottles for culture was used for aerobes, anaerobes, yeast, fungi, and mycobacteria. Specimen was not allowed to dry out. It was submitted wrapped in a sterile saline moistened (damp) nonadherent material. Transport and storage done at ambient room temperature. Appropriate antibiotic treatment was given as per the culture sensitivity report in the postoperative period for 8 weeks as suggested by the infection specialist. In patients with negative Gram stain and culture results, the treatment with an antimicrobial regimen with activity against the common causes of vertebral osteomyelitis, including staphylococci, streptococci, and gram-negative bacilli was ensured. An appropriate empiric regimen consisted of vancomycin plus one of the following: cefotaxime (2 g intravenous [IV] every 6 hours), ceftazidime (1 to 2 g IV every 8 to 12 hours), ceftriaxone (2 g IV daily), cefepime (2 g IV every 12 hours), or ciprofloxacin (400 mg IV every 12 hours or 500 to 750 mg orally twice daily). Anaerobes are uncommon pathogens in patients with vertebral osteomyelitis, and we do not routinely add anaerobic coverage to initial empirical therapy. Such coverage was warranted if clinical features suggest that the infection may be due to anaerobic organisms (such as in the setting of a concomitant intra-abdominal abscess) or if the Gram stain is positive but aerobic cultures are negative. In such cases, metronidazole (500 mg IV every 6 hours) may be added to the above regimen. Calcium and Vitamin D3 were given to all the patients. Postoperatively, all patients were mobilized as per their tolerance and advised to undertake physiotherapy. Those patients with suboptimal screw hold were braced with a lumbosacral corset for 3 months. The response to antibiotic therapy was judged by the declining values of inflammatory markers (CRP) and with an improving mobility/decreasing pain. Cultured organisms and histopathological reports of biopsy material were noted.Operating room time from incision to closure minutes was noted in minutes. Estimated blood loss (EBL), length of hospital stay (LOH) Oswestry Disability Index (ODI) score (preoperative to the index TELD surgery, preoperative to TLIF surgery, 6 weeks, 2 years at final follow-up) was used to quantify functional clinical outcome. For the ODI a change of minimum clinical interpretable difference of 11% was considered a significant improvement [25]. A patient satisfaction index was used as a self-assessment tool to determine the overall satisfaction outcome [26]. Clinical and radiological results were assessed at 6 weeks, 3 months, and 6 months and yearly afterwards. A follow-up period of minimum 2 years was considered for inclusion in outcome calculation. The final radiological outcome was accepted as stable and fused if no periscrew loosening/broken implant was present, and the stabilized segment showed the static position of cage with appreciable intercorporeal bone formation [27]. This was assessed on static and dynamic radiographs. The resumption of basic activities of daily living (ADL) with in house activities (in days) after the TLIF and resumption of previous activity/job (in months) were analyzed. Complications, if any were noted and managed accordingly. Failure to respond to treatment was considered a complete failure. All patients were reviewed regarding the time between onset and TLIF surgery, and the length of follow-up (months).
1. Literature Search StrategyThe publications covering the range of bacterial presentation in POSe were chosen using the PubMed search database. The terms "percutaneous," "transforaminal," "lumbar," "endoscopic," and "discectomy" were used in our search. Transforaminal, secondary spondylodiscitis, biopsy, and descriptions of culture organisms were the inclusion criteria for papers.
RESULTSAll 19 patients were operated with TELD as the first index surgery before presenting to our institute. There were 11 female patients (57.89%) and 8 male patients (42.10%) (the average age was 44.36±11.11 years). Patients’ demographic features are listed and summarized in Table 1. The average duration of the onset of POS symptoms was 24.42 days after the index surgery. All patients had persistent back pain; the most common level of the lesion in our study was L4–5 (n=10). It was noted that in the patients presenting to us after index TELD surgery. A biopsy was not undertaken before giving antibiotics in 15 patients, and all of those were operated by interventionist. Four cases who were operated by surgeons had biopsy and targeted treatment but did not respond. In the index surgery, 8 patients were operated within 10 days of the onset of symptoms. Out of these, 9 patients were operated on by interventionist and one by surgeon. At our center, all 19 patients were operated with open TLIF, and followed-up to 52.94±13.66 months. The mean duration of the operation was 69.47±17.39 minutes. Bone graft alone was used in 5 patients, and 14 cases were added with an interbody cage. There was 6 gram-positive and 4 gram-negative bacteria and 9 patients with no organism growth (Table 2). The surgical variables are tabulated (Table 3). The preoperative VAS for leg pain was 5.78±4.19 (6–10), which improved to 0.84±0.60 (0–2) at 2 weeks, then 0.94±0.62 (0–2) at 6 weeks and 0.52±0.61 (0–2) at final follow-up. The preoperative VAS for back pain was 9.47±0.61 (9–10) which improved to 1.47±1.02 (1–4) at 2 weeks then 0.31±0.47 (0–1) at 6 weeks and 0.42±0.50 (0–1) at final follow-up (VAS score bar diagram Figure 1). The preoperative ODI score was 87.01±7.70 (73.33–97.78) which improved to 7.36±8.14 (0–26.67) at final follow-up. The functional outcome is tabulated (Table 4) (Figure 2,3). The CRP was monitored for progressive reduction and normalization at 8 to 10 weeks in all the patients. In our cases all the culture-specific treated (n=10) and empirical treated (n=9), responded (100% infection cure). None needed a change of regimens and neither of the empirical cases warranted anaerobic treatment. The final radiological outcome was fusion with no screw loosening/broken implant, the stabilized segment showed the static position of cage with appreciable intercorporeal bone formation as assessed on static and dynamic radiographs. Three minor complications occurred. An incidental dural tear in 2 patients, which healed spontaneously. This was assessed on static and dynamic radiographs (100% fusion). A PubMed search of database results displayed 259 studies, out of which 5 were included.
DISCUSSIONThe total reported incidence of postoperative spinal infection varies from 0.7%–16% while POS incidence in MLD/OD is reported as 0%–3.7% [28-30]. POSe following FESS TELD is a rare complication. The reported POSe incidence in the methodological literature search we did as part of our study shows that it is very less and reported to be from 0.12% to 1.72% [18,31-34]. Infection can spread by hematogenous spread or by other inflammatory causes in immunocompromised patients [35-38]. Comorbid elderly, immunosuppression, renal failure, and diabetes mellitus are some of the risk factors that cannot be changed in POS pathogenesis, but obesity, smoking, invasive catheter, and prolonged hospital timing are the ones that can be changed [38-40].Thorough, improved irrigation mixed with saline, and antibiotics in TELD leads to reduced POSe [10,17]. Diagnosis can get delayed or difficult in early presentation, and a high index of suspicion is needed [37]. Diagnosing discitis in the postoperative spine is more challenging than detecting a primary spondylodiscitis [41]. MRI is reliable in early diagnosis of POSe though with many caveats [42,43].
Persistent severe back pain is present in most cases of POS [21]. Clinical features of POSe are like primary POS. Back pain and fever are usually predominant. Neurological deficits, and sphincter loss occurs occasionally. Pain is more at night rather than daytime. Physical examination shows localized spinal tenderness, muscle spasms, and limitations of spinal movements [18,40,44]. Elevation in the ESR and CRP are routine markers of spine infection. CRP is proven to be superior to ESR as it is reported that the reaction time of CRP is lesser than ESR [30,45]. The diagnosis of POS is often complex as its management, but it should be diagnosed on the accurate judgment of the surgeon/clinician [46]. All our patients had classic POS features (100%).The organisms found in POS after open surgery are mainly but not limited to; gram-positive aerobic cocci: Staphylococcus aureus, Streptococcus pyogenes, Coagulase-negative staphylococci, Other streptococci, Enterococcus spp; gram-negative aerobic bacilli: Escherichia coli, Proteus spp, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterobacter spp, Salmonella spp, Serratia marcescens; Anaerobic bacteria: Propionibacterium spp, Bacteroides fragilis, Pepto streptococcus spp [41,47,48]. There are few reports of published literature on POSe which mentions about the organism and are tabulated (Table 5) [17,31-34]. In most of the studies of TELD the type of organism was not reported in the literature [35,49,50]. In our series Mycobacterium tuberculosis (MTB) was present in 1 case. Three cases of POS due to MTB are reported in English literature [51-53]. It was noted that the cases of MTB culture-positive cases in our series were operated at rural centers for the index LDH surgery, based on poor quality MRI images of 0.5 Tesla machines. This was carried out within 10 days of the presentation without probably adequate conservative trial. In high probability, it can be speculated that it may be an early presenting tuberculous infection itself, which was not picked up by the MRI radiologist and operating interventionist. In our study also we found no different organism than the organisms in POSe reported in literature (Table 2) and regular POS.
Nosocomial seeding can be due to a lack of care in the sterility of the instruments and direct contamination of instruments [17,33]. Wrong placement of the needle or accidental bowel penetration with a steep angle and organism introduction while repositioning to disc target is a causal suggested by Ahn and Lee [17]. Direct skin contamination as the needle advances is a possibility. Rigid endoscopes are heat sensitive. Hence, the instrument cannot withstand the temperature. Therefore, using Ethylene oxide is the best method for the sterilization of rigid endoscopes [54]. Being more delicate instruments, nonautoclaving methods like cidex (glutaraldehyde) are frequently used [55]. Cidex is a solution that is effective in killing microorganisms from the surface of instruments and has a broad-spectrum antimicrobial activity and is reliable for killing vegetative bacteria, fungi, and viruses [56]. Before sterilizing the endoscopes, the endoscopes should be first dismantled, precleaned with disinfectant, and then dried [57]. There are guidelines suggested for optimizing endoscope reprocessing to achieve optimal outcomes [58,59]. Exposure to surgical draping, longer operation time, and continuous in-out movement of the endoscopes/image intensifier has also been held responsible for POSe [17,33]. Other causes may include frequent and multitude of instruments, external draping help from non-scrubbed assistants, irrigation contamination, and surgical glove perforation [17,33,44].
Biopsy and culture should be done before empirical treatment in POS, and positive result for the organism is obtained in 75%–80% of patients. The false negative culture can be present due to exposure to the antibiotics prior to biopsy. But biopsies performed after the antibiotics exposure shows positive result in only 50% of patients [40]. This was noted in our series also with nearly 47.37% (n=9) culture negativity. In cases of empirical treatment in these 9 cases also the outcome of infection cure was achieved. In a case of community acquired infection where culture sensitivity has not grown any organism, it is presumed to have common gram-positive and gram-negative organisms other than Methicillin-resistant Staphylococcus aureus. To cover these group of organisms, board spectrum 2nd and 3rd generation cephalosporins are good empirical choice to treat such community acquired infections in general [60]. Computed tomogrpahy-guided biopsy or trocar biopsy is commonly done [47,61,62]. Biopsy along with TELD itself as a management of lumbar infectious primary spondylodiscitis, is an effective treatment method in early cases (76%), and provide higher bacterial diagnostic efficacy (90%) [47].
POS is treated primarily with immobilization and a combination of antibiotics from 4 weeks to 24 weeks [61-65]. The timeline and best technique for treating POSe are still up for debate. The treatment plan for POS should be decided based on whether it’s early or late POS. A literature review by Rutges et al. [66] compares the conservative and surgical techniques for treating spondylodiscitis. The author reports that the antibiotic therapy is safe and effective in the early period of the infection when the antibiotics are given based on the targeted causative organism. Though, by giving the antibiotic therapy, an additional surgery was still required in 25%–55% of the reported cases of POS. The failure rate in the surgical outcome of POS was reported to be 2%–5% [66]. Surgical options for patients with POSe can vary from re-TELD debridement to fusion. TELD irrigation can provide immediate pain relief and a good outcome for patients with POS [6,36,47,67]. A key point should be noted that if the patient does not respond well to the antibiotic therapy in the early period, the surgeon should go for the surgical intervention because in the late period, the infection will affect the mechanical stability of the disc [17-18,33]. Mechanical dysfunction is in all probability the point of no return in the conservative care of POS. Patients’ disability may last well over few months before the natural history of spontaneous healing can be achieved [68]. In POSe or any POS, surgeons may have a less threshold for resurgery in a patient operated at another institute. An approach with more patience in their own operated patients is a possibility and they may strikingly continue protracted conservative care. In all the POSe patients (n=19), TLIF was operated by us but, they all were other institute operated index TELD surgery. We took the call for active intervention in the presented patients at an average of 24.42 duration days. All the patients were in a higher order of disability (ODI, 87.10; back pain VAS, 9.4). But strikingly no biopsy was previously done in the patients presented to us by the primary physician, again showing up the unrecommended empirical therapy approach which may not work. The recent consensus paper developed by a working group of the American Society of Pain and Neurosciences has commented that many of the procedures made for spine surgeons are becoming more facile after getting into the hands of interventionist [69]. Complications such as colitis, renal failure, and allergic reactions can also occur due to empirical antibiotics side-effects and antimicrobial resistance is another looming problem [70,71]. In our study, TLIF was done in all patients as it achieves a single-stage fusion through only a posterior approach. Recently, multiple retrospective studies have reported greater improvement in sagittal alignment with instrumentations [72]. TLIF technique may be the best familiar pathway to achieve complete debridement, access the disc space, remove an avascular disc, achieve circumferential fusion, and avoid the unfamiliar anterior approach [21,23,73-76]. In our series of 19 patients, we achieved reasonable mean operative time, EBL, less LOH, fewer complication rates, and quick improved back pain VAS (Tables 3, 4). The cured infection, fusion segment (100%), and improved ODI at average final follow-up of 52.94 months, proves TLIF to be a very reasonable approach for the management of POSe (Figure 1).
None of the studies in POS mention the resumption of basic ADL or going back job timeline [39,62,63,77,78]. Resumption to basic activities and job was assessed in our study and reasonable early return were noted in all patients. Patients with disc space infection progress to spontaneous interbody fusion within a period of 6 to 12 months [61,66]. The conservative treatment should be given for not more than 3–4 weeks because delaying the ambulation and productivity can have long-lasting impact on the patient in various forms of disability, and psychological dysfunction as a burden of cost. Multiple studies from the literature have supported the aggressive surgical approach in managing these patients [22,24,65]. Earlier in orthopedics and spine, the use of implants in the presence of infection was greatly feared. However, there is now abundant literature that supports the safe utilization of implants even in the presence of infection. [79-81].
FESS needs standards of endoscope reprocessing and surgical re-training to be followed stringently [56,67]. That is why its suggested for all surgeons and pain interventionists to follow the same standards. To maximize the relevance of this study to the general spine surgical practice the author recommends, an approach of repeat TELD in early weeks of POSe with biopsy specific antibiotic treatment. In failure to respond by relief in pain and infection markers in a further fortnight, especially if a mechanical pain has set in, then a decision of fusion should not be delayed. Early inappropriate action, inaction, not offering indicated surgery, implicit bias, subjective decision making rather than objectivity, all will pave the way for Artificial intelligence in near future [82].
There are many limitations to this study. It can be argued that the study was conducted in a surgical population with the very small numbers in this study. True incidence of POSe cannot be calculated. But equally or bigger number of patients may be getting better at the hands of other surgeons and interventionist with primary or secondary POS surgeries or with even empirical treatments. This study did not examine the details of antibiotic treatments, neither given empirically, nor the ones given after the fusion. This was not a complete vertical study. The details of the index surgery diagnosis, images and the indications for surgery were not assessed as it was not the focus of the study. But it was noted that one patient with lytic spondylolisthesis was operated on by TELD by interventionist, again pointing to deficiencies of training and interpretation of images (Figure 2, 3). In our series, the index surgery of TELD in 8 patients were operated within 10 days of onset of symptoms. Out of these, 9 patients were operated on by interventionist and one by surgeon. This again points towards non-standardized practice, and under attempted duration of conservative trial.
CONCLUSIONWith the use of increasing TELD by surgeons and interventionist in clinical practice, there is also an increase in related complications though very less like POSe. It needs biopsy-based specific treatment. In nonresponsive cases, TLIF executed timely can reduce the ordeal of long sufferings.
ACKNOWLEDGEMENTSThe authors thank the Stavya research team for assistance with manuscript preparation.
Figure 1.Bar graph representing the preoperative and postoperative scores. VAS, visual analogue scale; ODI, Oswestry Disability Index. 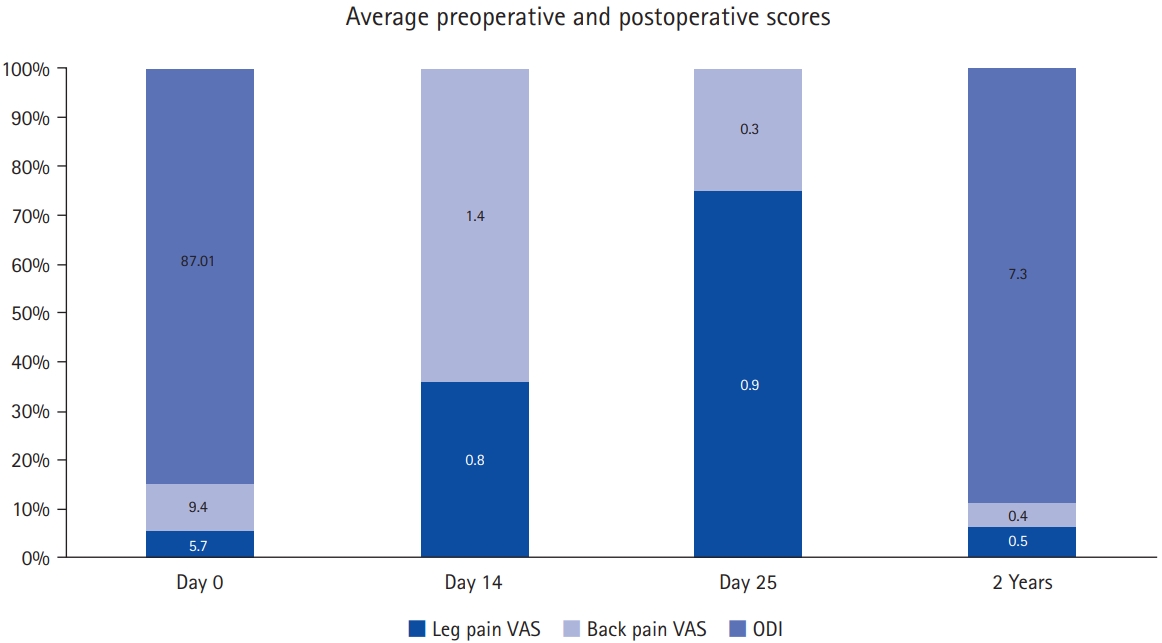
Figure 2.(A) T2-weighted sagittal magnetic resonance imaging (MRI) of a female patient with an annular bulge at L4–5. Transforaminal endoscopy in the index surgery. (B–D) T2-weighted sagittal, T1-weighted sagittal, and T2-weighted axial MRI showing an endoscopic view at 3 weeks postoperatively of spondylodiscitis with end plate changes, collapsing disc space, vertebral body edema and wet facets. (E) Surprisingly, the radiograph showed lytic at the L5 level, and the patient underwent surgery primarily without a dynamic radiograph by the interventionalist physician. 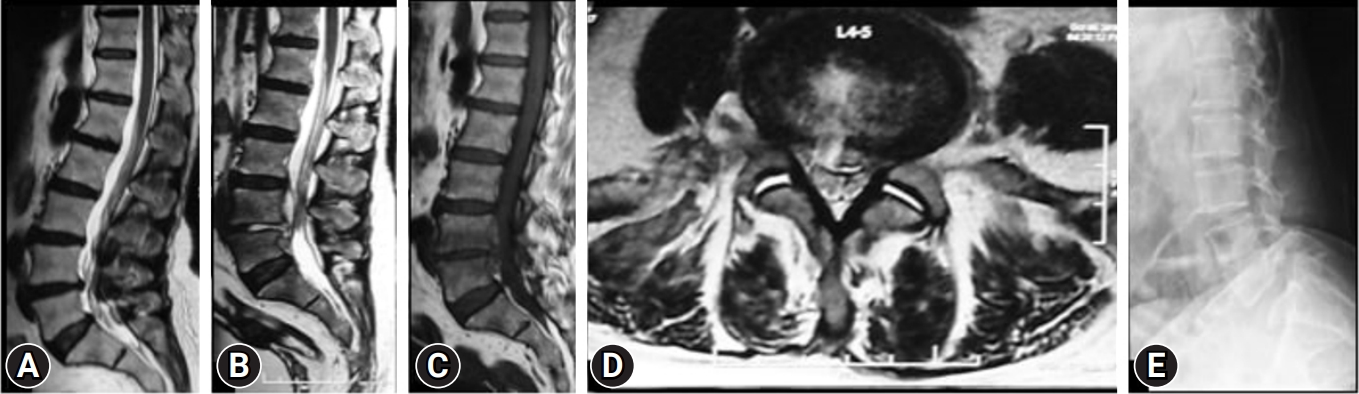
Figure 3.(A, B) Anteroposterior and lateral radiograph of the patient in Figure 1, who then underwent transforaminal lumbar interbody fusion with bone grafting and culture-specific antibiotic treatment. (C, D) One-year anteroposterior and lateral radiograph showing uniting healing at L4–5. (E, F) Five-year follow-up anteroposterior and lateral radiographs, showing consolidated stable reconstruction with nonprogressive L5 lytic. The clinical result was excellent with occasional back pain due to lytic. 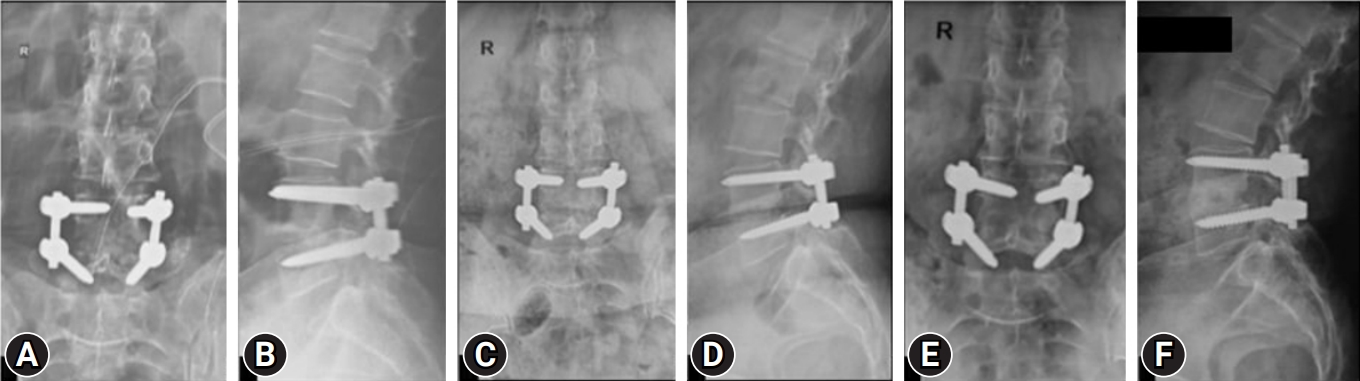
Table 1.Demographic features of patients Values are presented as mean±standard deviation (range) or number (%). BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; CAD, coronary artery disease; IHD, ischemic heart disease; THY, hypothyroidism; RA, rheumatoid arthritis; TB, tuberculosis; LBP, low back pain, RLP, radicular leg pain; TELD, transforaminal endoscopy lumbar discectomy; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate. Table 2.Culture results in patients with their frequency (n=19) Table 3.Surgical variables, satisfaction outcome variables, and complications Table 4.Postoperative functional outcome scores Table 5.Published literature on POSe
REFERENCES1. Chung AS, McKnight B, Wang JC. Scientific view on endoscopic spine surgery: can spinal endoscopy become a mainstream surgical tool? World Neurosurg 2021;145:708–11.
2. Akbary K, Kim JS. Recent technical advancements of endoscopic spine surgery with disparate or disruptive technologies and patents. World Neurosurg 2021;145:693–701.
3. Lewandrowski KU. "Outside-in" technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: a retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int J Spine Surg 2014;8:26.
4. Li XC, Zhong CF, Deng GB, Liang RW, Huang CM. Full-endoscopic procedures versus traditional discectomy surgery for discectomy: a systematic review and meta-analysis of current global clinical trials. Pain Physician 2016;19:103–18.
5. Ruan W, Feng F, Liu Z, Xie J, Cai L, Ping A. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: a meta-analysis. Int J Surg 2016;31:86–92.
6. Ito M, Abumi K, Kotani Y, Kadoya K, Minami A. Clinical outcome of posterolateral endoscopic surgery for pyogenic spondylodiscitis: results of 15 patients with serious comorbid conditions. Spine (Phila Pa 1976) 2007;32:200–6.
7. Choi G, Modi HN, Prada N, Ahn TJ, Myung SH, Gang MS, et al. Clinical results of XMR-assisted percutaneous transforaminal endoscopic lumbar discectomy. J Orthop Surg Res 2013;8:14.
8. Kapetanakis S, Gkantsinikoudis N, Thomaidis T, Charitoudis G, Theodosiadis P. The role of percutaneous transforaminal endoscopic surgery in lateral recess stenosis in elderly patients. Asian Spine J 2019;13:638–47.
9. Zhu Y, Zhao Y, Fan G, Gu G, Sun S, Hu S, et al. Comparison of the effects of local anesthesia and epidural anesthesia for percutaneous transforaminal endoscopic discectomy in elderly patients over 65 years old. Int J Surg 2017;48:260–3.
10. Krishnan A, Kohli R, Degulmadi D, Mayi S, Ranjan R, Dave B. Cauda Equina Syndrome: a review of 15 patients who underwent percutaneous transforaminal endoscopic lumbar discectomy (PTELD) under local anaesthesia. Malays Orthop J 2020;14:101–10.
11. Namboothiri S, Gore S, Raja PT. Novel Surgical Technique for discogenic cauda equina syndrome-transforaminal intra discal access by annulotomy outside central spinal canal. J Spine 2016;S7:2.
12. Lee DY, Lee SH. Learning curve for percutaneous endoscopic lumbar discectomy. Neurol Med Chir (Tokyo) 2008;48:383–8.
13. Ahn Y, Lee SH, Park WM, Lee HY, Shin SW, Kang HY. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine (Phila Pa 1976) 2004;29:E326–32.
14. Taylor DG, Buchholz AL, Sure DR, Buell TJ, Nguyen JH, Chen CJ, et al. Presentation and outcomes after medical and surgical treatment versus medical treatment alone of spontaneous infectious spondylodiscitis: a systematic literature review and meta-analysis. Global Spine J 2018;8(4 Suppl):49S–58S.
15. Pan M, Li Q, Li S, Mao H, Meng B, Zhou F, et al. Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician 2020;23:49–56.
16. Kim HS, Sharma SB, Wu PH, Raorane HD, Adsul NM, Singh R, et al. Complications and limitations of endoscopic spine surgery and percutaneous instrumentation. Indian Spine J 2020;3:78–85.
17. Ahn Y, Lee SH. Postoperative spondylodiscitis following transforaminal percutaneous endoscopic lumbar discectomy: clinical characteristics and preventive strategies. Br J Neurosurg 2012;26:482–6.
18. Krishnan A, Barot MP, Dave BR, Bang P, Devanand D, Patel D, et al. Percutaneous transforaminal endoscopic discectomy and drainage for spondylodiscitis: a technical note and review of literature. J Orthop Allied Sci 2018;6:16–20.
19. Kulkarni AG, Hee HT. Adjacent level discitis after anterior cervical discectomy and fusion (ACDF): a case report. Eur Spine J 2006;15 Suppl 5(Suppl 5):559–63.
20. Bavinzski G, Schoeggl A, Trattnig S, Standhardt H, Dietrich W, Reddy M, et al. Microsurgical management of postoperative disc space infection. Neurosurg Rev 2003;26:102–7.
21. Shetty AP, Aiyer SN, Kanna RM, Maheswaran A, Rajasekaran S. Pyogenic lumbar spondylodiscitis treated with transforaminal lumbar interbody fusion: safety and outcomes. Int Orthop 2016;40:1163–70.
22. Salgotra B, Attry S, Patel D, Sansiya K. Open transforaminal lumbar interbody fusion (TLIF) for post-discectomy spondylodiscitis: our experience. IP Indian J Neurosci 2018;4:144–9.
23. Liao JC, Chen WJ. Revision surgery for postoperative spondylodiscitis at cage level after posterior instrumented fusion in the lumbar spine-anterior approach is not absolutely indicated. J Clin Med 2020;9:3833.
24. Chang CW, Tsai TT, Niu CC, Fu TS, Lai PL, Chen LH, et al. Transforaminal interbody debridement and fusion to manage postdiscectomy discitis in lumbar spine. World Neurosurg 2019;121:e755–60.
25. Yoshida G, Hasegawa T, Yamato Y, Kobayashi S, Shin O, Banno T, et al. Minimum clinically important differences in Oswestry Disability Index domains and their impact on adult spinal deformity surgery. Asian Spine J 2019;13:35–44.
26. Toyone T, Tanaka T, Kato D, Kaneyama R, Otsuka M. Patients' expectations and satisfaction in lumbar spine surgery. Spine (Phila Pa 1976) 2005;30:2689–94.
27. Dave BR, Samal P, Sangvi R, Degulmadi D, Patel D, Krishnan A. Does the surgical timing and decompression alone or fusion surgery in lumbar stenosis influence outcome in Cauda Equina Syndrome. Asian Spine J 2019;13:198–209.
28. Parchi PD, Evangelisti G, Andreani L, Girardi F, Darren L, Sama A, et al. Postoperative spine infections. Orthop Rev (Pavia) 2015;7:5900.
29. Rohde V, Meyer B, Schaller C, Hassler WE. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine (Phila Pa 1976) 1998;23:615–20.
30. Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. Acta Neurochir (Wien) 1995;136:145–50.
31. Kim WJ. Pyogenic psoas abscess and secondary spondylodiscitis as a rare complication of percutaneous endoscopic lumbar discectomy: a case report. Jt Dis Relat Surg 2005;16:163–6.
32. Sharma R, Basit MA, Zaid F. Incidence of discitis in endoscopic spine surgery. Int J Health Sci 2022;6(S3):10248–53.
33. Choi KB, Lee CD, Lee SH. Pyogenic spondylodiscitis after percutaneous endoscopic lumbar discectomy. J Korean Neurosurg Soc 2010;48:455–60.
34. Yörükoğlu AG, Göker B, Tahta A, Akçakaya MO, Aydoseli A, Sabancı PA, et al. Fully endoscopic interlaminar and transforaminal lumbar discectomy: analysis of 47 complications encountered in a series of 835 patients. Neurocirugia (Astur) 2017;28:235–41.
35. Zhu B, Jiang Y, Shang L, Yan M, Ma HJ, Ren DJ, et al. Complications of percutaneous endoscopic lumbar discectomy: experiences and literature review. J Spine 2017;402:321–7.
36. Wang X, Zhou S, Bian Z, Li M, Jiang W, Hou C, et al. Unilateral percutaneous endoscopic debridement and drainage for lumbar infectious spondylitis. J Orthop Surg Res 2018;13:306.
37. Grane P, Josephsson A, Seferlis A, Tullberg T. Septic and aseptic post-operative discitis in the lumbar spine--evaluation by MR imaging. Acta Radiol 1998;39:108–15.
38. Fantoni M, Trecarichi EM, Rossi B, Mazzotta V, Di Giacomo G, Nasto LA, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci 2012;16 Suppl 2:2–7.
39. Kucuk A, Karademir M, Tumturk A, Ulutabanca H, Ercal BD, Senol S, et al. Surgical strategies for spondylodiscitis due to lumbar disc surgery. Turk Neurosurg 2017;27:95–8.
40. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010;65 Suppl 3:iii11–24.
41. Dufour V, Feydy A, Rillardon L, Redondo A, Le Page L, Bert F, et al. Comparative study of postoperative and spontaneous pyogenic spondylodiscitis. Semin Arthritis Rheum 2005;34:766–71.
42. Yeom JA, Lee IS, Suh HB, Song YS, Song JW. Magnetic resonance imaging findings of early spondylodiscitis: interpretive challenges and atypical findings. Korean J Radiol 2016;17:565–80.
43. Gerometta A, Bittan F, Rodriguez Olaverri JC. Postoperative spondilodiscitis. Int Orthop 2012;36:433–8.
45. Grollmus J, Perkins RK, Russel W. Erythrocyte sedimentation rate as a possible indicator of early disc space infection. Neurochirurgia (Stuttg) 1974;17:30–5.
46. Almansour H, Pepke W, Akbar M. Pyogenic spondylodiscitis: the quest towards a clinical-radiological classification. Orthopade 2020;49:482–93.
47. Yang SC, Fu TS, Chen LH, Chen WJ, Tu YK. Identifying pathogens of spondylodiscitis: percutaneous endoscopy or CT-guided biopsy. Clin Orthop Relat Res 2008;466:3086–92.
48. Giordan E, Marton E, Scotton G, Canova G. Outcomes and risk factors for spontaneous spondylodiscitis: case series and meta-analysis of the literature. J Clin Neurosci 2019;68:179–87.
49. Kim MJ, Lee SH, Jung ES, Son BG, Choi ES, Shin JH, et al. Targeted percutaneous transforaminal endoscopic diskectomy in 295 patients: comparison with results of microscopic diskectomy. Surg Neurol 2007;68:623–31.
50. Gu YT, Cui Z, Shao HW, Ye Y, Gu AQ. Percutaneous transforaminal endoscopic surgery (PTES) for symptomatic lumbar disc herniation: a surgical technique, outcome, and complications in 209 consecutive cases. J Orthop Surg Res 2017;12:25.
51. Lotfinia I, Vahedi P. Late-onset post-diskectomy tuberculosis at the same operated lumbar level: case report and review of literature. Eur Spine J 2010;19 Suppl 2(Suppl 2):S226–32.
52. Jeon DW, Chang BS, Jeung UO, Lee SJ, Lee CK, Kim MS, et al. A case of postoperative tuberculous spondylitis with a bizarre course. Clin Orthop Surg 2009;1:58–62.
54. Parsons DW, Lee NL, Preminger GM. Care and sterilization of instruments. In: Smith AD, Preminger G, Badlani GH, Kavoussi LR, editors. Smith’s textbook of endourology. 2nd ed. Ontario (BC): Decker; 2007. p. 7–10.
55. Babb JR, Bradley CR. Endoscope decontamination: where do we go from here? J Hosp Infect 1995;30 Suppl:543–51.
56. Rutala WA, Weber DJ. Disinfection of endoscopes: review of new chemical sterilants used for high-level disinfection. Infect Control Hosp Epidemiol 1999;20:69–76.
57. Oh HJ, Kim JS. Clinical practice guidelines for endoscope reprocessing. Clin Endosc 2015;48:364–8.
58. Thornhill G, David M. Endoscope-associated infections: a microbiologist's perspective on current technologies. Tech Gastrointest Endosc 2019;21:150625.
59. Tandon RK, Ahuja V. Non-United States guidelines for endoscope reprocessing. Gastrointest Endosc Clin N Am 2000;10:295–318.
60. Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: An updated review. Int J Infect Dis 2019;81:128–36.
61. Krishnan A, Marathe N, Degulmadi D, Mayi S, Rai RR, Kumar S, et al. Transpedicular vertebral biopsy under O-arm navigation: a technical note. Egyp J Neurosurg 2022;37:1–6.
62. Rajasekaran S. Spinal infections & rrauma. New Delhi (India): Jaypee Brothers Medical Publishers; 2011..
63. Basu S, Ghosh JD, Malik FH, Tikoo A. Postoperative discitis following single-level lumbar discectomy: our experience of 17 cases. Indian J Orthop 2012;46:427–33.
64. Adam D, Papacocea T, Hornea I, Croitoru R. Postoperative spondylodiscitis. A review of 24 consecutive patients. Chirurgia (Bucur) 2014;109:90–4.
65. Santhanam R, Lakshmi K. A Retrospective analysis of the management of postoperative discitis: a single institutional experience. Asian Spine J 2015;9:559–64.
66. Rutges JP, Kempen DH, van Dijk M, Oner FC. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: a systematic literature review. Eur Spine J 2016;25:983–99.
67. Choi EJ, Kim SY, Kim HG, Shon HS, Kim TK, Kim KH. Percutaneous endoscopic debridement and drainage with four different approach methods for the treatment of spinal infection. Pain Physician 2017;20:E933–40.
68. Hadjipavlou AG, Katonis PK, Gaitanis IN, Muffoletto AJ, Tzermiadianos MN, Crow W. Percutaneous transpedicular discectomy and drainage in pyogenic spondylodiscitis. Eur Spine J 2004;13:707–13.
69. Naidu RK, Chaturvedi R, Engle AM, Mehta P, Su B, Chakravarthy K, et al. Interventional spine and pain procedure credentialing: guidelines from the american society of pain & neuroscience. J Pain Res 2021;14:2777–91.
70. Mann S, Schütze M, Sola S, Piek J. Nonspecific pyogenic spondylodiscitis: clinical manifestations, surgical treatment, and outcome in 24 patients. Neurosurg Focus 2004;17:E3.
71. Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 2014;6:25–64.
72. Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech 2002;15:149–56.
73. Ahsan K, Hasan S, Khan SI, Zaman N, Almasri SS, Ahmed N, et al. Conservative versus operative management of postoperative lumbar discitis. J Craniovertebr Junction Spine 2020;11:198–209.
74. Wang B, Chen C, Hua W, Ke W, Lu S, Zhang Y, et al. Minimally invasive surgery oblique lumbar interbody debridement and fusion for the treatment of lumbar spondylodiscitis. Orthop Surg 2020;12:1120–30.
75. Kim SH, Kang MS, Chin DK, Kim KS, Cho YE, Kuh SU. Anterior lumbar interbody fusion for the treatment of postoperative spondylodiscitis. J Korean Neurosurg Soc 2014;56:310–4.
76. Wu S, Lin B, Li X, Chen S, Zhang H, Wu Z, et al. Single-stage debridement via autogenous iliac bone graft through the OLIF corridor and lateral fixation in treating spontaneous single-level lumbar pyogenic spondylodiscitis. BMC Musculoskelet Disord 2021;22:947.
77. Moon MS, Kim SS, Lee BJ, Moon JL, Sihn JC, Moon SI. Pyogenic discitis following discectomy. J Orthop Surg (Hong Kong) 2012;20:11–7.
78. Das S, Mahmood E, Alam MJ, Rashid MM, Mitra PK. Management of postoperative discitis: a study on 20 cases of prolapsed lumber intervertebral disc (PLID) operation in a tertiary level hospital in Bangladesh. J Dhaka Med Coll 2014;23:186–90.
79. Khanna K, Janghala A, Sing D, Vail B, Arutyunyan G, Tay B, et al. An analysis of implant retention and antibiotic suppression in instrumented spine infections: a preliminary data set of 67 patients. Int J Spine Surg 2018;12:490–7.
80. Hey Hwee WD, Ng Li WN, Kumar N, Lau Tze-Chun E, Joseph T, Naresh K, et al. Spinal implants can be retained in patients with deep spine infection: a cohort study. J Orthop Trauma Rehabil 2018;24:34–8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||