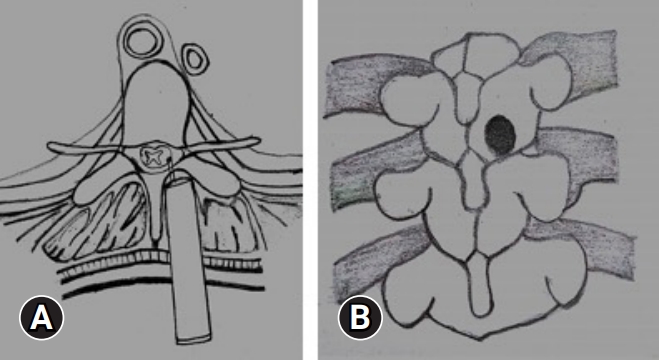
INTRODUCTION
Thoracic spinal stenosis is less commonly seen in spinal practice, as compared to cervical and lumbar levels. Of the various causes like thickening/ossification of ligamentum flavum (OLF), thoracic disc herniation, ossified posterior longitudinal ligament and tumors, OLF is the most common cause of chronic symptoms [1]. Rarity of this disorder may also be due to frequent misdiagnosis. Reported cases are more common in middle aged men [2]. The standard treatment of such patients with symptomatic thoracic spinal stenosis is decompression by open laminectomy. Other methods include laminoplasty with or without fusion and laminectomy with fusion. A forewarning of these methods is that they can influence the stability of the spine and scar the paraspinal muscles. Microscopic tubular decompression (MTD) by unilateral laminotomy for bilateral decompression can resect the OLF while preserving the spinous processes with the supra and interspinous ligaments, the cranial part of each lamina, and the lateral facets. Hence, the method is supposedly superior to conventional wide laminectomy in that the posterior structures of the spine are preserved. Treatment of this condition by microendoscopic decompression is reported by only few authors [3-6]. Here we present a small case series of 12 patients of thoracic spinal stenosis treated by MTD and followed up for a minimum of 1 year. The outcomes were evaluated in terms of improvement of Nurick grading and intraoperative/postoperative complications.
MATERIALS AND METHODS
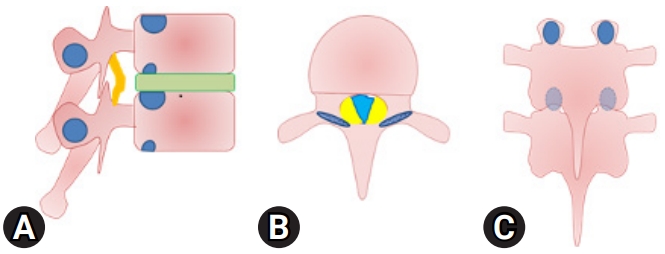
Twelve patients with symptomatic thoracic spinal stenosis with or without co-existing spinal stenosis at other regions (lumbar or cervical) were included in the study. Inclusion criteria were patients with complaints of numbness or paresthesia below affected level, gait difficulties, back pain, hyperreflexia and increased muscle tone upon clinical examination with magnetic resonance imaging (MRI) evidence of ligamentum flavum thickening resulting in thoracic canal stenosis. The whole spine was evaluated for tandem stenosis. Patients with other causes of thoracic spinal stenosis such a disc herniation or a tumor and multiple-level thoracic spinal stenosis (Figure 1) were excluded from the study.
Demographic data in terms of age, sex, co-existing illness etc. were collected. The duration of symptom onset and the level/s affected were also taken into consideration. The preoperative disability of the patients in terms of Nurick grading [7] were recorded and improvement noted after the surgery were also recorded in terms of Nurick grading. A computed tomography (CT) scan of the dorsal spine was also done in all the patients to identify the morphology of the OLF, its site and extent and note the presence of a dural ossification. The MRI was thoroughly evaluated to plan the target and the extent of decompression. Also, the levels of OLF to be addressed in a case of multilevel affection were also decided on prudent observation of MRI and clinical correlation with the signs and symptoms. Any change in the signal intensity of the spinal cord at the affected level was also noted. The presence of ossified dura, associated ossification of the posterior longitudinal ligament and any other anterior compressive element were investigated for in MRI. No evoked potentials were used in all procedures. The operative time, amount of blood loss, and duration of postoperative hospital stay were evaluated. Intraoperative and postoperative complications in terms of wound infection, hematoma collection, dural tear, root injury, cord injury etc. were also observed for. The patients were followed up for a minimum period of 1 year to evaluate the improvement in disability. A postoperative CT scan and MRI were also done in a few patients to evaluate the extent of decompression achieved.
1. Case Example
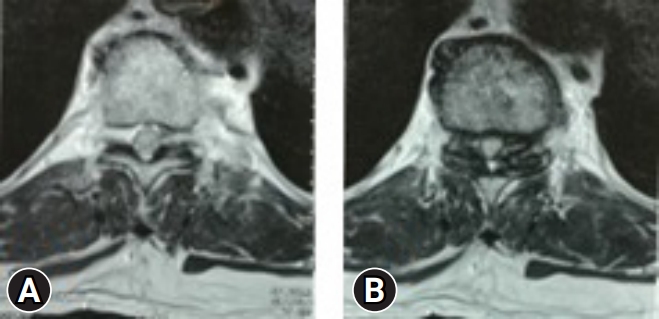
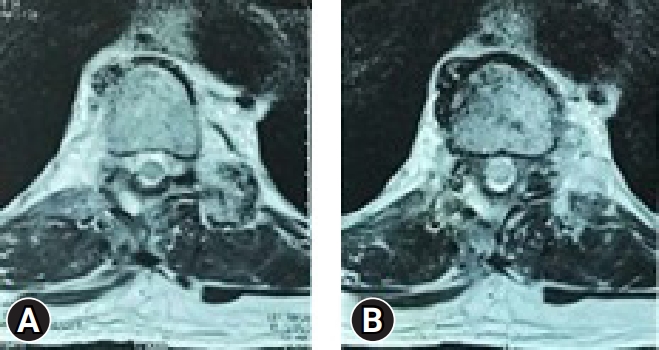
A 67-year-old male came with complaints of gait disturbance and difficulty in walking since 2 months. He was able to walk with difficulty even with aid of walker. He had numbness in both lower limbs. His bowel and bladder were unaffected. Examination revealed a positive Rhomberg test, exaggerated reflexes, and normal power in upper limbs. His myelopathy was scored as Nurick 4. MRI evaluation revealed a stenosis at T10–11 level (Figure 2). He had some degenerative changes at lumbar level, but there were no symptoms or signs related to it. Hence, it was decided to decompress the affected level by MTD (Figure 3).
2. Operative Procedure
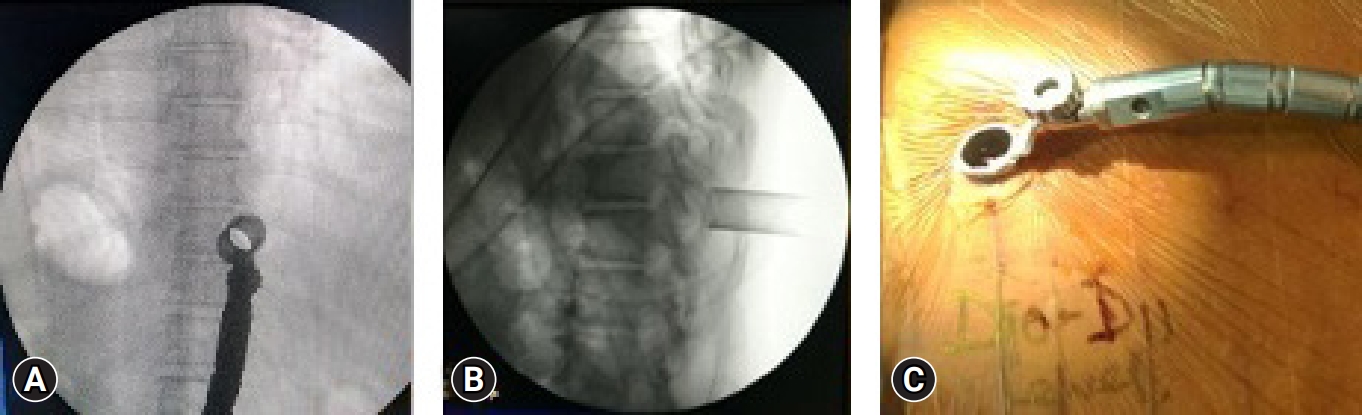
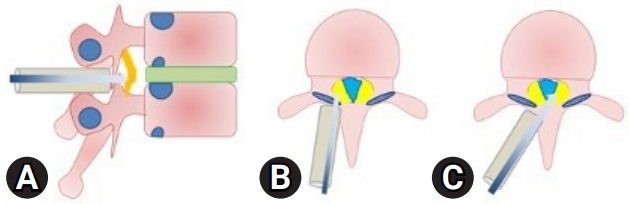
After positioning of the patient in prone, the level is localized under C-ARM. A spinal needle is introduced at that level and infiltrated with a mixture of 2% lignocaine and adrenaline. An 18-mm paracentral incision, 1 cm from the midline is taken and serial dilatation up to 18 mm using tubular dilators is done and a tubular retractor is docked (Figure 4). We perform a laminotomy at the affected level, preserving the facet joint, using a pneumatic burr and a microscope (Figure 5). The lateral margin of decompression is the pedicle or lateral edge of the dural sac. To avoid neural injury during the contralateral decompression, one must preserve the ligamentum flavum covering the thecal sac on the ipsilateral side. Then the contralateral side is addressed by manipulating and angling the tube and microscope. For contralateral decompression the “wishbone” portion of the cephalad and caudal lamina, i.e., the junction of the lamina with the spinous process should be resected (Figure 6). Then the contralateral ligamentum flavum is resected. Sometimes, a dura dissector is used to peel off the underlying OLF off the dura. The same procedure is done on the ipsilateral side. By repositioning, angling, and manoeuvring the tubular retractor and microscope, we were able to obtain a wide view of the spinal canal with proper illumination and thus increasing the safety of the procedure.
RESULTS
The mean age of the patients was 59 years. There were 9 males and 3 females. The average duration of symptoms was 8.6 months. The average follow-up period was 15.4 months.
1. Patient Assessment
All the patients had single affected vertebral level in the lower thoracic spine. The patients were evaluated in terms of Nurick grading presurgery and postsurgery at final follow-up. The improvements are tabulated below (Table 1).
2. Outcome
The most commonly affected level noted in the present study was T8–9. The mean preoperative Nurick score was 3.83 while the mean postoperative Nurick score was recorded as 1.5. An obvious improvement in the outcome was noted in terms of Nurick scoring.
3. Intra- or Postoperative Complications
There were no serious postoperative complications. All patients were mobilized out of bed with or without assistance on the same day of surgery. An aggressive physiotherapy protocol, tailor made to each patient was administered and strict adherence was reinforced. One patient had a postoperative hematoma on day 4 after the surgery, as evidenced by power loss and increasing numbness. It was confirmed with a postoperative MRI. The hematoma was evacuated through same incision. The final outcome was uneventful. However, the patient required a prolonged hospital stay.
DISCUSSION
OLF is a well-known cause of progressive thoracic myelopathy. Plogar first reported OLF using lateral radiographs in the 1920s; Yamaguchi described it as thickened/ossified ligamentum flavum [8]. It commonly involves lower thoracic spine (T9–12) with upper thoracic spine (T1–4) being the next common site which are the junctional areas [9]. The natural course and prognosis of OLF are still unclear, but good surgical results are expected even in patients who already show myelopathy [2]. The duration of preoperative symptoms and preoperative severity of myelopathy have been shown to be important factors in the prognosis [10].
Open surgical decompression with laminectomy has been considered the gold standard for patients suffering from the disabling symptoms of thoracic stenosis [11,12]. It gives an excellent and wide field for excision of the pathology. In multilevel cases, extensive removal of the posterior elements may destabilize the spine necessitating instrumentation and fusion. Laminoplasty in these cases may be tried, but is technically difficult in those cases where the dura is adherent to the ligamenta flava [12]. Okada et al. [13] reported several patients in whom post laminectomy deterioration secondary to increased kyphotic deformity of the thoracic spine. The resection of the posterior elements and the facet joints compromises spinal stability. Hence, a minimally invasive approach using a tubular retractor system preserves the posterior elements and theoretically reduces the chance of instability. Microendoscopic decompression aims to expand the canal diameter by unilateral laminotomy approach for bilateral decompression. Microendoscopic decompression has been widely used in the treatment of lumbar spinal stenosis. In this respect, applications Microscopic Tubular Decompression to the thoracic spine may be a useful alternative to traditional open decompression.
The intraoperative docking of the tube at the disc level is particularly important. Unlike the lumbar spine or the cervical spine, localization of the correct level is often tricky in the thoracic spine and wrong level exploration is a possibility [14]. The advantages of the tubular retractor in minimizing the incidence of wrong level surgery are well established [15]. The tube is docked such that the region from the inferior aspect of the superior pedicle (for example, T9 in case one is performing T9–10 decompression) to the superior aspect of inferior pedicle (T10) is within the confines of the tube. This will allow decompression of that part of the canal in the region of the facet joints which is the site of stenosis. Unlike in the lumbar spine where due skilful diligence is required in ensuring a thorough decompression of the canal without harming the integrity of the facet joints, the stability provided by the rib cage allows the surgeon to decompress more liberally in the region of the thoracic facet joints to ensure adequate decompression [16]. The short disc heights in the thoracic spine as well as the degenerative changes in the segment further add to the stability of the segment in spite of an overzealous decompression. The laminotomy begins medially and is extended laterally onto the facet joint until the lateral margins of the dura are visualized. An excellent visualization of the surgical field is obtained with the magnification and illumination provided by the microscope.
However, the thoracic canal is narrower than the lumbar canal. Subsequently, the work corridor is less and careful handling of instruments and the drill becomes imperative. Also, while in lumbar region, where one just has to retract the dura during the procedure, in the thoracic region, due to the presence of the spinal cord; one has to be careful and meticulous while manipulating the instruments through the corridor.
The estimated blood loss, operative time, hospital stay, postoperative pain in our series were similar to that seen in lumbar MTD. Thus, the technique offers several advantages over conventional open procedures. The use of tubular retractor minimizes muscle dissection and muscle trauma. During the procedure we undercut the inferior spinous process, leaving the posterior tension band in place and thus minimizing disruption to the supraspinous ligament, interspinous ligament, and paraspinal muscle. Preservation of these structures hypothetically reduces the possibility of the development of kyphotic deformity. Thus, this technique aims to achieve an adequate decompression of the neural structures while minimizing surgical damage to the posterior stabilizing structures. It preserves the facet joints as well as the neural arch of the contralateral side to shield the neural structures against posterior scarring. It also preserves the ligamentous structures that function as a mechanical strut in the movement of the spine, thereby allowing improved postoperative muscle function. Unlike lumbar, in thoracic the decompression is at spinal cord level so this technique needs high degree of accuracy and learning curve. Since MTD is highly demanding, multilevel decompression through tubes will causes surgeon fatigue. Also, it is not advisable in cases where there is an associated dural ossification because of technical difficulties with associated high risk of dural rupture
CONCLUSION
In patients with thoracic spinal stenosis secondary to OLF, the goal of surgical decompression to remove the hypertrophied tissue safely and effectively can be achieved by MTD. MTD allows a magnified surgical field while minimizing the disruption to surrounding soft tissue and bone structures and allows complete decompression of the spinal cord with minimal alteration to the biomechanical strength of the vertebral column. The alleged advantages of tubular decompression in lumbar levels viz. decreased blood loss, reduced hospital stay, early mobilization, less muscle injury etc. are also applicable in this scenario. Rapid recovery from the surgical treatment is a potential advantage of this approach. The high prevalence of postoperative back pain associated with conventional total laminectomy with or without fusion could be dodged by this approach. However, long-term follow-up is needed to confirm these results as every decompressive procedure bears the risk of secondary instability and being a single center retrospective analysis, it may not be reproductible, and more studies should be suggested.