AbstractObjectiveTo demonstrate the details of the far-lateral approach (FLA) as a minimally invasive technique for the excision of the upper cervical anterolateral and anterior meningiomas and dumbbell schwannomas, and to assess the clinical and radiological outcomes.
MethodsIn this technical report and case series we report the FLA technique and patients who underwent the FLA for C1-C4 anterolateral and anterior meningiomas and dumbbell schwannomas between June 2007 and June 2020. All patients’ relative preoperative demographic, clinical, radiographic, operative, histopathological, and perioperative complications and follow-up clinical and radiographic data were reported.
ResultsA total of 19 patients including 12 females and 7 males with a mean age 56.7±17.6 years and mean duration of symptoms 12.8±12.3 months were reported. 9 patients with anterolateral meningiomas, 5 with anterior meningiomas, and 5 with dumbbell schwannomas underwent uneventful FLA procedures. Gross total resection of tumors was reported in 17 patients (89.5%). Preoperative JOA score was normal in ten, grade-I in five, and grade-II in 4 patients, while at the last follow-up it improved to normal in seventeen and grade-I in two patients. Reported postoperative JOA scores at 6 months and at the last follow-up showed that all patients improved at least one grade on JOA scores. There was CSF leak in three patients and superficial wound infection in one.
INTRODUCTIONIntradural extramedullary upper cervical tumors are mostly benign for which advances in imaging modalities and recent microsurgical techniques yielded better clinical outcome and prognosis [1,2]. Most of these tumors are meningiomas and schwannomas. Although they are benign lesions, they represent a surgical challenge, particularly the anterolateral or anterior lesions representing 55% of cervical meningiomas [1] and hourglass or dumbbell lesions representing 50% of cervical schwannomas [3-5]. As the goal of surgery is total resection with preservation of function, stability, and acceptable morbidity, several surgical techniques were introduced. The posterior midline, far-lateral approach (FLA), posterior unilateral hemilaminectomy, and rarely the anterior approaches are selected to be used depending on either the location of the tumors or the preference and experience of the surgeon [5-10]. While the first and fourth techniques are conventional, the second and third are considered minimally invasive techniques.
Although the posterior midline approach is standard and familiar to most surgeons, it carries the risk of cord manipulation and affecting cervical spine muscle stability. Meanwhile, the FLA is direct surgical access with low or no risk of cord manipulation, preserving cervical spine stability, muscles, and ligaments thus considered minimally invasive, and finally ensuring smooth total tumor removal. The FLA had been designed first for foramen magnum lesions by George et al. [11] and then extended by Salas et al. [12] who introduced a variety of technical modifications or variations to suit different pathologies, and finally, it has been extended to the upper cervical spine down to C4 by other surgeons [1,7,13].
Few series in the literature have focused only on managing the upper cervical anterolateral and anterior meningiomas and dumbbell schwannomas using the FLA. Therefore, the primary purpose of the current study is to demonstrate the surgical details of the technique of the FLA as a minimally invasive technique for excision of the upper cervical anterolateral and anterior meningiomas and dumbbell schwannomas. The secondary purpose is to assess the clinical and radiological outcomes in patients who underwent surgery.
MATERIALS AND METHODSThis study has been approved by our Institutional Ethical and Research Review Board (IRB No. 4616#). In this technical report and retrospective case series, we report our technique that has been performed on patients who underwent surgery using the FLA for upper cervical anterolateral and anterior meningiomas and dumbbell schwannomas. Patients with tumors extending from C1 to C4, with complete clinical, radiographic, and contact data and those who complete at least one-year follow-up were included in this report. This study has been carried out at the spine unit of Neurosurgical Department, Suez Canal University Hospital. Our hospital records were reviewed through the period from 2007 to 2020 searching for all patients that have been operated on via the FLA for upper cervical lesions. Patients treated through other approaches, other tumor locations, other pathologies, and foramen magnum tumors and those with incomplete data were excluded. Nineteen patients were eligible for reporting in this study after the exclusion of 7 patients due to incomplete data and/or follow-up.
Surgical excision of tumors was categorized as gross total resection (GTR) when grossly excised, subtotal resection (STR) when some tumor remnants were left attached to the dura or nerve root, and partial resection when a bulky mass was left.
All patients’ relative preoperative demographic, clinical, radiographic, operative, histopathological, and perioperative complications and follow-up clinical and radiographic data were reported. All patients formally provided their consent prior to surgery, and the study has been approved by our institutional ethical and research review board.
All patients were submitted for anteroposterior and lateral plain radiographs and T-1 and T-2 weighted MRI of the cervical spine with gadolinium enhancement in the sagittal, coronal, and axial views. Patients with dumbbell schwannomas were further submitted to multi-slice CT scan.
1. Operative TechniqueUnder general anesthesia, the patient was placed in the lateral decubitus position and the head was slightly flexed and contralaterally tilted and fixed in Mayfield head clamp. The skin incision was marked one finger breadth behind the tip of the mastoid process and extended in a straight fashion according to the target cervical level (Figure 1, 2A). After opening the skin and deep fascia, the wound was deepened bluntly with the index finger between the sternomastoid muscle anteriorly and trapezius muscle posteriorly (Figure 2B). We continued deepening the wound bluntly in the facial plan between the scalene muscles anteriorly and levator scapulae muscle posteriorly targeting the tip of the atlas transverse process above and the lateral mass of the cervical vertebrae below depending on the intended target cervical level (Figure 2C). For C1 and C2 vertebrae after identifying the tip of the transverse process of C1, the inferior oblique muscle was stripped of the tip of the spinous process of C2 and dissected and retracted anteriorly protecting the vertebral artery (Figure 2D). Meanwhile, for C2, C3, and C4 vertebrae, the paraspinal muscles were striped subperiosteally of the lateral masses and the laminae by a Cobb muscle elevator exposing the laminae between the facet joints and the base of the spinous processes of the corresponding vertebrae (Figure 3A). Working posterior to the facet joint after exposing the hemilaminae of the target level, a laminotomy of these laminae using a high-speed drill and a Kerrison rongeur from the fact joints anteriorly to the base of the corresponding spinous process posteriorly was conducted (Figure 3B). After full dural exposure, a median vertical dural incision was performed, and anterior dural edge was stitched to the ipsilateral muscles to expose the tumor, with the posterior edge remained in situ to protect the spinal cord.
Carrying out this step will directly expose the tumor just beneath the anterior dural edge in the visual access of the surgeon, while the spinal cord is still covered and protected by the posterior part of the dura (Figure 3C). The dentate ligaments could be sharply cut allowing tumor access and relaxing the spinal cord. At this stage, tumor debulking was performed through a combination of thermal bipolar coagulation, rongeurs, curettes, and ultrasonic aspirator (Figure 3D). We usually start dissecting the tumor from the anterior dura, where the tumor originated, using bipolar coagulation. This makes the tumor almost avascular facilitating its removal and/or debulking. Some meningiomas are soft and could be easily sucked through the suction tube apparatus, allowing internal cavitation of the lesion and facilitating tumor mobilization with little cord manipulation. Some are tough, so in these cases we can use ultrasonic aspirator.
In small or medium sized tumors, bipolar coagulation could be utilized to cauterize the plan between the dura and tumors down to the opposite side and hence remove the de-vascularized tumor in one piece. After tumor excision, adequate hemostasis was conducted and the dural base was cleaned and/or cauterized with bipolar coagulation to avoid any tumor recurrence. At this stage, the anterior and contralateral dura is in the direct visual access of the surgeon while the spinal cord is posteriorly covered and protected by the posterior dural sleeve (Figure 4A).
1) In Case of Dumbbell Schwannomas (Figure 5)After the usual muscle and bony exposure according to the target level, the extradural intraforaminal part of the lesion will come up directly into the visual access of the surgeons between the thinned-out bones of the adjacent vertebrae (Figure 6A). Hemilaminectomy of the adjacent vertebrae was conducted for more exposure of the dural sleeve of the foraminal component as well as the dura of the spinal cord (Figure 6B). The dura covering the intraforaminal part was opened, and excision of that part was performed first. After complete excision of the foraminal part, the proper spinal dura was opened either in normal vertical linear fashion or in transverse fashion as an extension of the foraminal dural opening (Figure 7A). After exposing the intradural part, we check the mass mobility and locate the rootlets from which the lesion originates and try to preserve or sacrifice it according to the circumstances (Figure 7B). Neurophysiological monitoring can ensure safety of tumor removal and protect against operative neurologic deficits especially in large sized tumors.
2) Wound Closure and Postoperative CareA watertight dural closure was then ensured while dural patch could be utilized in some cases (Figure 4B). The wound was closed in multilayer watertight fashion to obliterate any dead space starting with suturing the deep muscles of the posterior triangle. The scalene muscles and levator scapulae muscle are sutured, followed by suture approximation of the sternomastoid and trapezius muscles and then watertight sutures of the aponeurosis, and finally the subcutaneous tissue and skin are sutured. An epidural closed suction drain was inserted with its tube passing through the paraspinal muscle and not through wound to allow watertight closure of the fascia. The drain was observed and if no CSF leak was seen, it was removed 24 to 48 hours postoperatively. If CSF continued to leak through the drain, it was removed, and wound was observed and if a wound bulge appears, a lumbar CSF drainage through a lumbar puncture could be used. An MRI with Gadolinium enhancement was requested for the patient to document complete tumor removal and spinal cord signal and decompression. Patient was discharged from the hospital and scheduled for outpatient’s clinic.
2. Statistical AnalysisThe Statistical Analysis was performed using IBM SPSS version 25 soft wear (IBM Corp., Armonk, NY, USA). All continuous data are presented as a mean and standard deviation and were tested for normal distribution using the Kolmogorov–Smirnov test. Difference in baseline data and radiologic parameters were analyzed using the t-test or the Mann–Whitney U-test for continuous variables and the chi-square test or Fisher’s exact test for the categorical variables. Statistical significance was set at p<0.05.
RESULTSThe mean age of our 19 patients was 56.7±17.6 years (range, 39–60 years), including 12 females and 7 males. The major symptoms included gait disturbance in nine, radiculopathy in seven, and urinary symptoms in three with mean duration of symptoms 12.8±12.3 months (range, 3–18 months) (Table 1). At presentation, the 17-point Japanese Orthopedic Association scale (JOA scale) [14] indicated that ten patients were grade 0, five were grade-I, four were grade-II, and none were grade-III. Comorbidity included five hypertensive patients, four diabetic patients, and one rheumatoid arthritis patient.
Overall, nine patients have anterolateral meningiomas, five anterior meningiomas, and five dumbbell schwannomas. Considering the tumor level, the tumor localization is shown in Figure 8. All reported lesions were considered as large tumor as they occupied >50% of the spinal canal on axial MRI. Thirteen patients conducted their surgery from the left side and six from the right side depending on the lateralization of the lesion (Table 2).
All patients in this series underwent their surgical procedures uneventfully without any postoperative neurological deterioration. The summary of perioperative parameters, including operative time, operative blood loss, and hospital stay is shown in Table 2. The mean follow-up period was 29.72±19.80 months (12–52 months). GTR of tumors was reported in 17 patients (89.5%), and STR was reported in two patients out of 19 patients. STR patients included one with large dumbbell schwannoma, where a small part of the intraforaminal component could not be removed, and one with anterior meningioma, where a small part that was tethered to the anterior surface of the spinal cord and could not be removed. Both patients showed no regrowth during the period of follow-up. A patch dural graft was utilized for closing the dura in one schwannoma patient. Histopathological examination of the excised specimens revealed that eight specimens (57.1%) were psammomatous meningiomas, four (28.6%) were fibroblastic meningiomas, and two (14.3%) were meningothelial meningiomas. All excised meningiomas were WHO grade-I.
Reported postoperative JOA scores at 6 months and at the last follow-up showed that all patients improved at least one grade on JOA scores (Figure 9). There was no reported perioperative mortality in this series, while reported morbidity included CSF leak into the suction drain in three patients and superficial wound infection in another one. In patients with CSF leak into the suction drain, we kept the drain until leak stopped or if it continued for a week, we just removed it at that time, and this maneuver prevented wound CSF leak.
DISCUSSIONUpper cervical anterolateral and anterior meningiomas and dumbbell or hourglass schwannomas are not uncommon benign intradural extramedullary tumors. Relative to the dentate ligament, cervical meningiomas are frequently anterolateral, less frequently posterior, and rarely anterior to the dentate ligament and hence to the cervical cord. Overall, 44% of the dumbbell schwannomas are located in the cervical region, and up to 50% of the upper cervical schwannomas are dumbbell [3-5]. Due to the capacious spinal canal, lack of intervertebral foramina, and wide interspace between the atlas and the axis, schwannomas are more frequently huge or dumbbell-shaped than other spine locations [4]. They represent a surgical challenge due to their peculiar anatomical characteristics, extraspinal or anterior location to the spinal cord, and proximity to important vital structures like the vertebral artery and upper cervical spinal cord. Lots of surgical techniques and approaches have been designed to preserve neural function and stability, decrease morbidity, and improve outcome [5-8,10,15,16]. Apart from the standard posterior approach, unilateral hemilaminectomy approach, and anterior or anterolateral approach, the FLA as a minimally invasive technique is one of the attractive approaches designed to overcome all other approaches’ shortcomings.
In this case series and technical report, we demonstrated our technique and reported our experience of the FLA in managing upper cervical anterolateral and anterior meningiomas and dumbbell schwannomas. Twelve females and 7 males with a mean age 56.7 years with anterolateral meningioma in 9, anterior meningioma in 5, and dumbbell schwannomas in 5 patients were reported. All patients underwent the procedures without neurological deterioration, mortality, or significant morbidity.
In agreement with our results, Aboul-Enein et al. [13] reported 16 cases of ventrally located cervical meningiomas operated on via the FLA with GTR in 15 patients, no significant morbidity, and recurrence in 3 cases. They highlighted the advantages of their technique in preserving neural function and spinal stability. Wang et al. [8] reported 10 males and 8 females with a mean age 48.2 years who underwent FLA for C1-C2 dumbbell schwannomas with outcome similar to ours. They reported GTR in 15 and STR in 3, with one death due to pulmonary embolism and one recurrence in the 4-year follow-up. They reported that the FLA provided adequate exposure and access with minimal neural manipulation and considered the preferred approach for resection of ventrally or ventrolaterally located tumors.
Lot and George [9] reported 12 C1-C2 dumbbell neuroma cases who underwent posterolateral approach with GTR and reported good neurological results in most patients while preserving stability. Gu et al. [16] were able to perform GTR in 28 out of 35 dumbbell schwannomas through the posterior approach with unilateral facetectomy without affecting the stability. They resected the extradural component first as we preferred in our study. Their results were suboptimal in comparison with ours or other far lateral series [8,9]. McCormick [17] operated upon 12 patients with dumbbell cervical tumor including 9 schwannomas through the posterior approach with unilateral facetectomy and highlighted the issues of stability and root section. They concluded that the root section resulted in a nonsignificant deficit although it should be preserved, and unilateral facetectomy did not affect stability although it should be preserved.
Kim and Chung [18] reported 6 large ventrally located cervical schwannomas and 2 meningiomas via the posterior approach with GTR in all and without morbidity. Despite the satisfactory clinical outcome, they highlighted the drawbacks of the posterior approach. The facet joint is preserved to maintain spinal stability, and this limits the surgical exposure requiring a very meticulous microsurgical technique to manipulate vulnerable neural structures. Extraspinal tumor extension, meningiomas en plaque and tumors that tend to adhere to the spinal cord such as psammomatous meningiomas, calcified meningiomas, and recurrent tumors are major limitations of the posterior approach and make GTR sometimes impossible [19,20]. In this case series, 57% of meningiomas were psammomatous, which tend to adhere to adjacent neural structures. However, with the FLA technique, we could easily create a smooth plan of cleavage between tumor and spinal cord, and we achieved GTR in all but one. This highlights the importance of improved visual and surgical access of the FLA in such lesions. We did not encounter a calcified, recurrent, or en plaque one in our series.
Technical IssuesThe main advantages of the FLA as a minimally invasive technique are improved visual access to the area ventral to the spinal cord without neural retraction, the feasibility of accessing extradural extension, a safe and better manipulation of the plan between tumor and spinal cord, not destabilizing the spine by preserving the ipsilateral facet joint while the contralateral facets and posterior and anterior elements are untouched, not requiring spinal fixation, and not necessitating vertebral artery manipulation as the lesions are intradural [1,7,11,21]. This is in contrast to the extreme lateral approach where the condyles are violated and vertebral artery is manipulated in most variants of the approach except the retrocondylar variant [12,22].
In their trial to increase the advantages and decrease shortcomings of the standard posterior approach in managing the anterolaterally located meningiomas and schwannomas, some authors [23,24] have introduced some technical modifications in order to improve the surgical access to the tumor. Chang [23] presented a posterior paramedian approach as a simple versatile technique for obtaining lateral viewing angle to the cervical spine. Joaquim et al. [24] presented a modified technique that comprises tenting of the dentate ligament and rotation of the spinal cord in order to increase the small field and allow cord retraction of natural component. Slin’ko and Al-Qashqish [25], in their large series of ventral and ventrolateral tumor operated on using different techniques, reported that since the introduction of the anterolateral and dorsolateral approach, all patients had a safe GTR of their lesion with excellent recovery in most cases.
Lonjon et al. [1] highlighted the importance of FLA in resecting ventral tumors especially hard one while suggesting that the dorsolateral approach may be enough in soft suckable ventral tumors. However, it is still difficult to precisely determine tumor consistency and vascularity despite recent MRI study contribution in this issue such as fluid-attenuated inversion recovery (FLAIR), magnetic resonance elastography (MRE) [26,27], and arterial spin-labeling MRI [28].
The well-known skin incisions for the FLA are the inverted hockey stick incision [29], C-shaped incision [12], and linear paramedian incision [1,7,30]. In addition to the argument that the linear vertical incision decreases the risk of CSF leak, it reduces muscle trauma, provides excellent retraction in the transverse access, creates deep surgical field, and reduces risk of skin necrosis, pseudomeningocele, and fluid collection [31].
Although endovascular embolization may be associated with spinal cord swelling or ischemia, and venous bleeding, it could reduce intraoperative tumor hemorrhage that obscures vision and may preclude GTR was advocated in complex meningiomas [1]. We did not use embolization, and our operative blood loss was not significant and GTR was achieved in 89.5%. Hence, we believe that preoperative embolization is not mandatory in these lesions.
During our follow-up in this series, we have no tumor recurrence or regrowth on the two STR patients. This might be due to our high rate of GTR. According to Komotar et al. [22], tumor recurrence highly depends on the extent of tumor removal. However, most common causes for STR in FLA were never related to lack of exposure but rather adherence to surrounding structures, size of tumor, and prior radiation therapy or surgery [7,22].
This retrospective study has some limitations related to its retrospective nature and relative rarity of reported pathologies and surgical technique. The relatively small number of study populations and short follow-up period are other limitations. In such a situation, a multicenter study is highly recommended.
NOTESFig. 1.A 52-year-old female patient presented with Brown-Sequard Syndrome. Sagittal T2 (A) and T1(B), and post-contrast T1 MRI sagittal (C), coronal (D), and axial (E) showing large C2-C3 left anterolateral meningioma compressing the spinal cord. 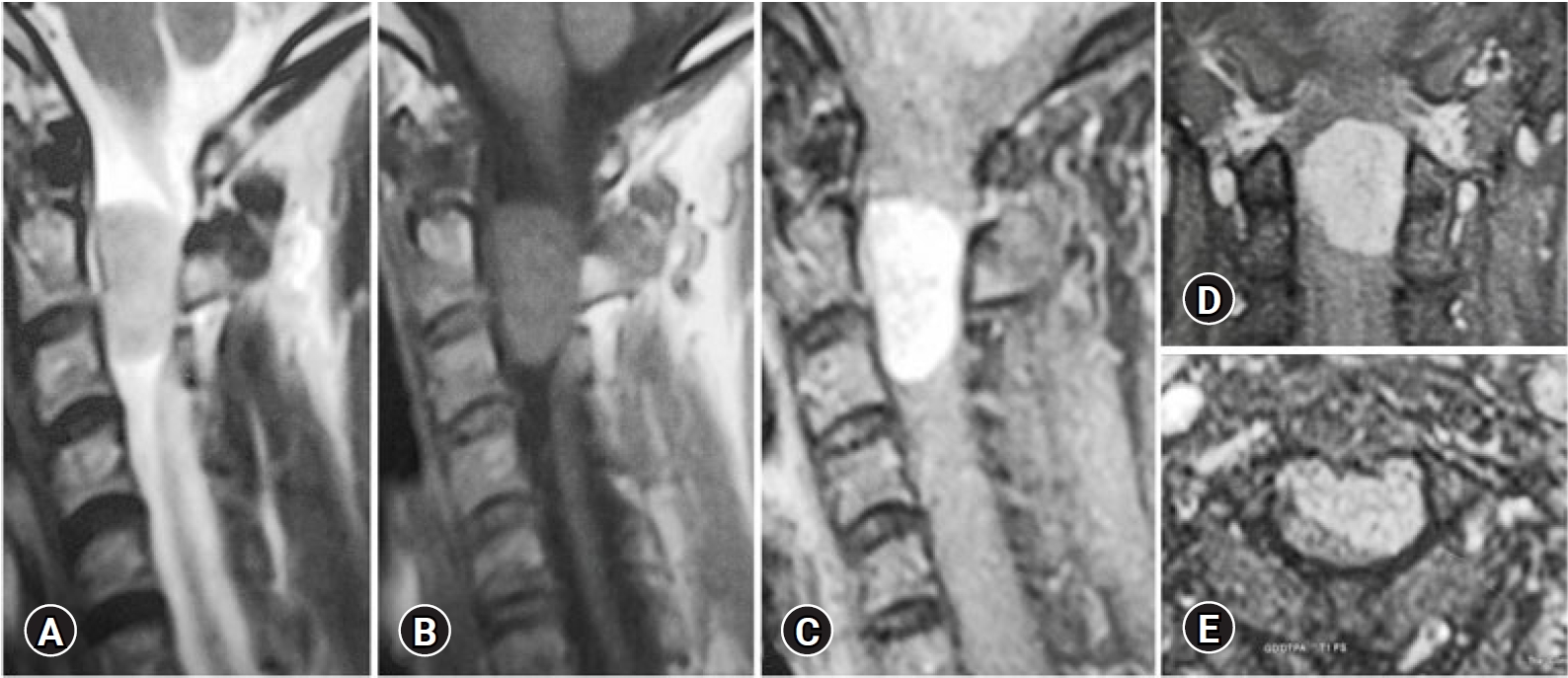
Fig. 2.(A) A linear skin incision finger breadth behind the mastoid opposite the index level. (B) Surgical exposure between the sternocleidomastoid (SCM) and trapezius (TM) muscles and then between the scalene muscles (SM) anteriorly and levator scapulae muscle (LM) posteriorly with fat pad (FB) in between that guide us to the deep muscle layer. (C) Exposure of the inferior oblique muscle (IOM) deep to the fat pad. (D) Section of the IOM and superior oblique (SOM) and exposure of C2 lamina. 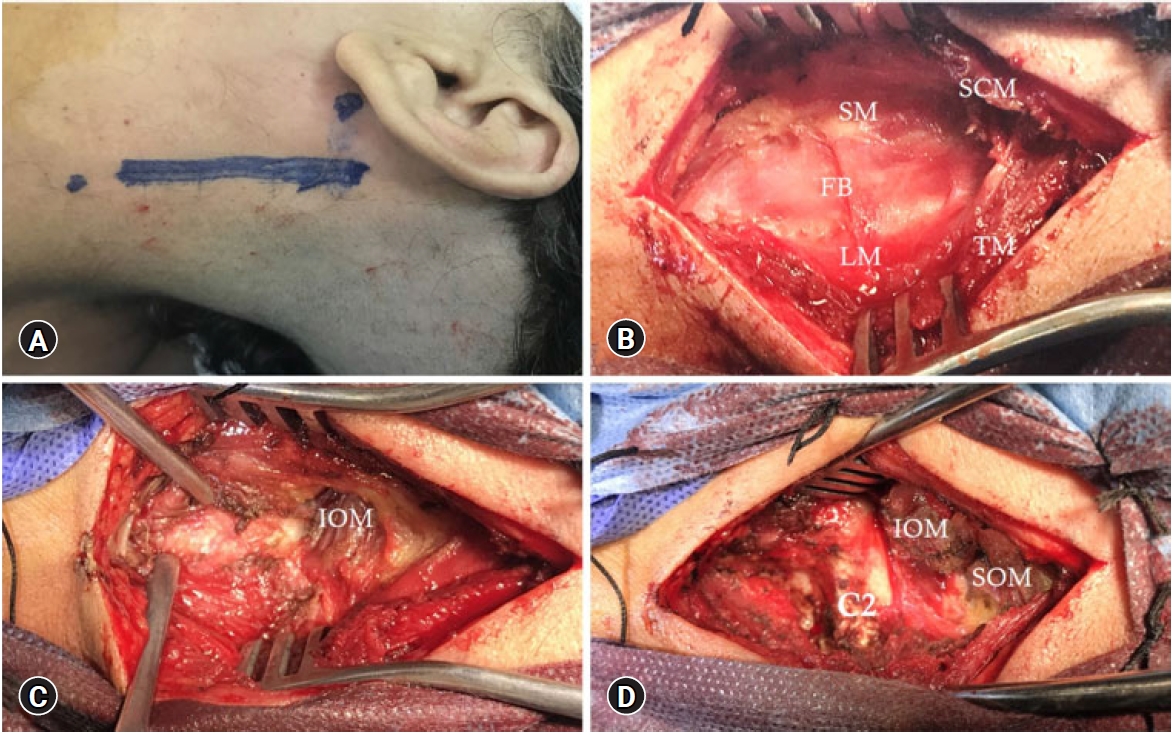
Fig. 3.(A) Exposure of C2 lamina and facet (FC2), C3 lamina and facet (FC3), and C1 lamina. (B) C2-C3 laminotomy exposing the spinal dura (D). (C) Opening the spinal dura and tenting the anterior dural edge exposing the meningioma (T) crossed by sensory rootlets. (D) Progressive tumor resection. 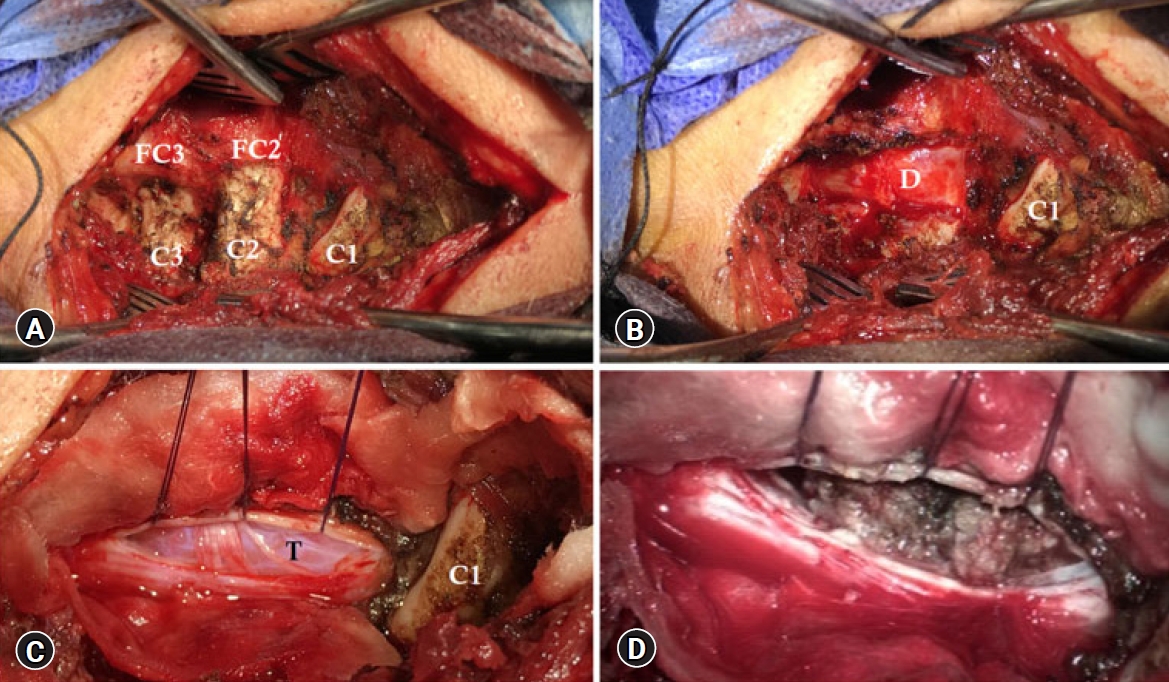
Fig. 4.(A) Tumor bed (TB) is shown with the contralateral spinal dura. (B) The dura was sutured directly. (C) 3D CT scan showing the extent of bone removed during the approach. 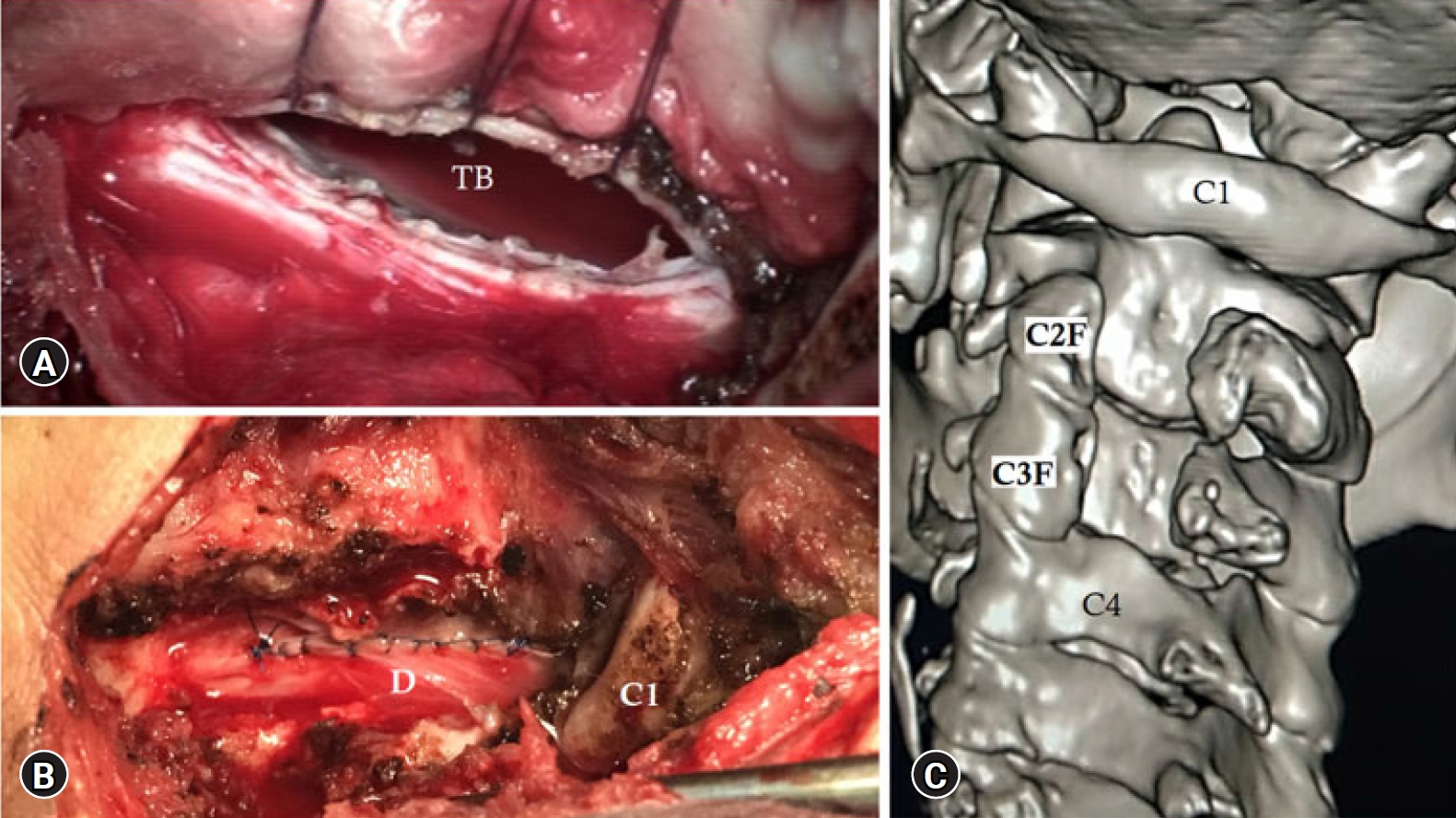
Fig. 5.A 44-year-old male patient presented with left occipital pain and gait disturbance. Sagittal T2 (A) and T1 (B), and post-contrast coronal T1 (C) MRI showing large C1-C2 dumbbell schwannoma with large intraformational component compressing the cervical spinal cord. 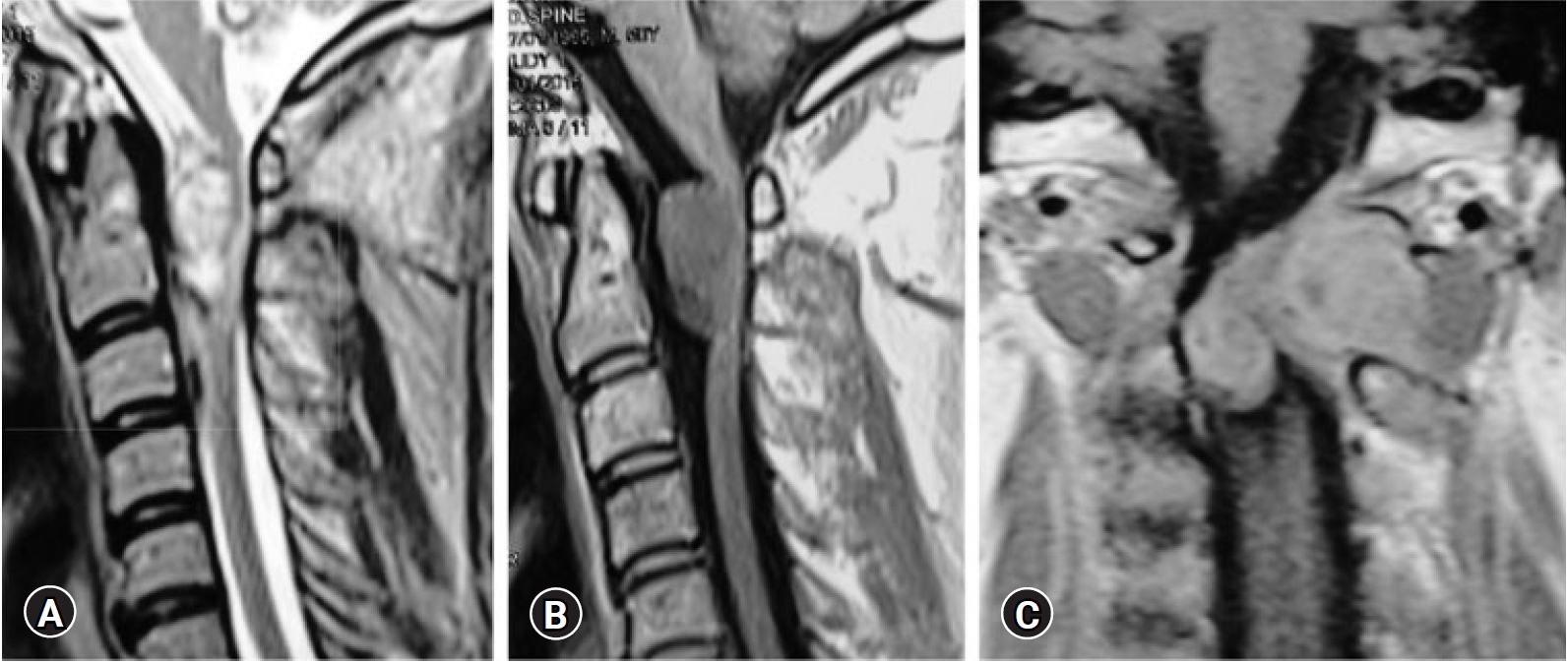
Fig. 6.(A) Bony exposure showing the intraformational part of the schwannoma (DS) between the thinned out C1 and C2 laminae. (B) After C1-C2 laminotomy showing C2 facet (C2F), remains of atlas lamina (C1), and the intraforaminal dumbbell schwannoma (DS) compressing the spinal cord dura (D). 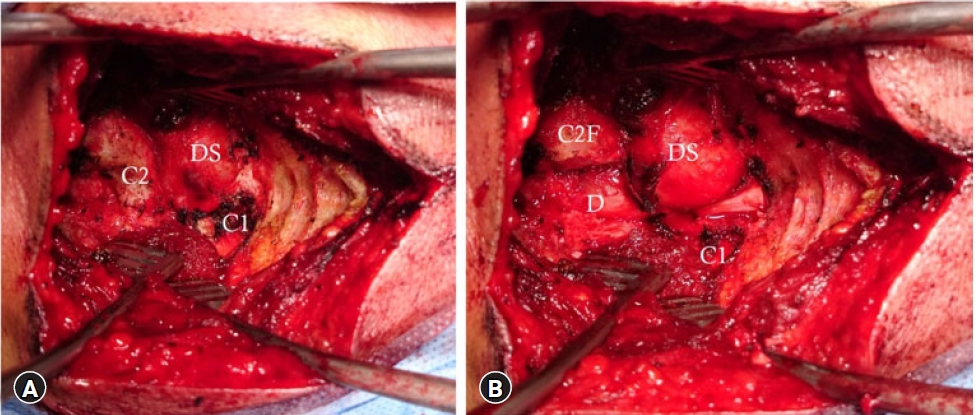
Fig. 7.(A) After removal of the intraforaminal part and opening the spinal dura (D) exposing the intradural dumbbell schwannoma (IDS). (B) Tumor removed showing the contralateral spinal dura (CD). 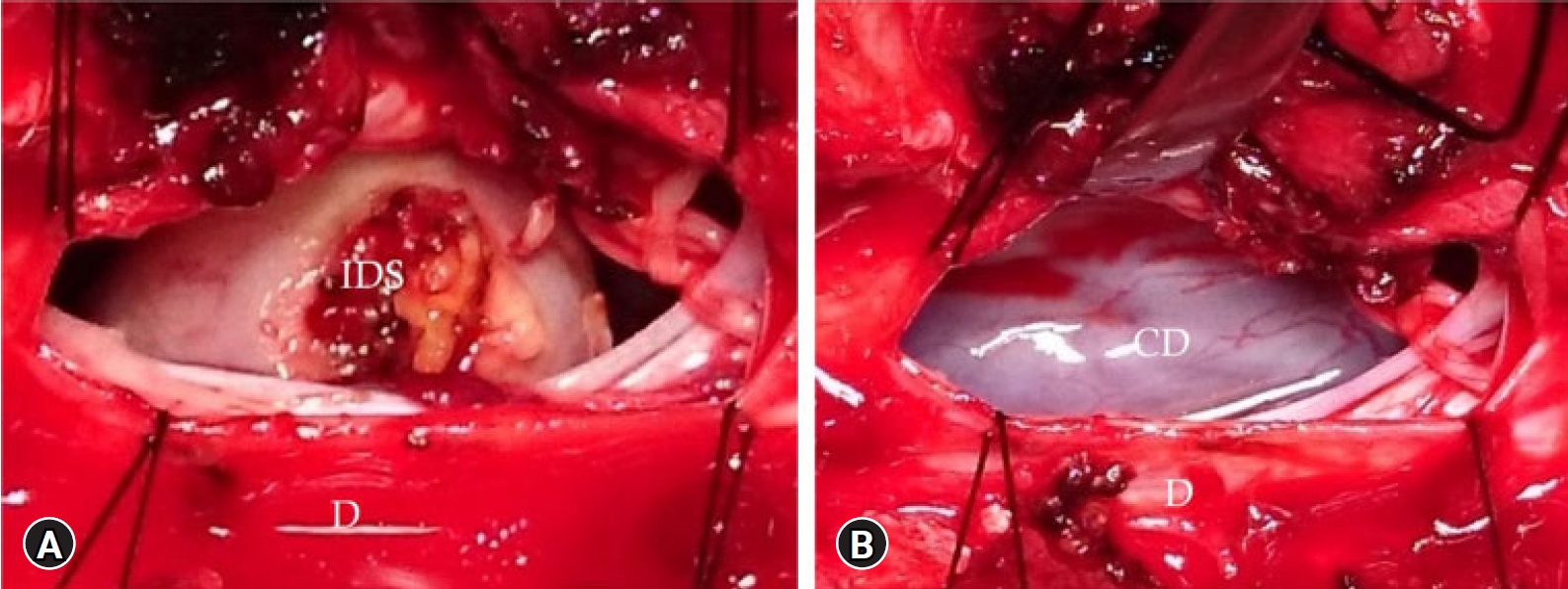
Table 1.Data summary of reported patients (N=19) Table 2.Perioperative parameters (N=19) REFERENCES1. Lonjon N, Russo V, Barbarisi M, Choi D, Allibone J, Casey A. Spinal cervical meningiomas: the challenge posed by ventral location. World Neurosurg 2016;89:464–473.
2. Gezen F, Kahraman S, Canakci Z, Bedük A. Review of 36 cases of spinal cord meningioma. Spine (Phila Pa 1976) 2000;25:727–731.
3. Ozawa H, Kokubun S, Aizawa T, Hoshikawa T, Kawahara C. Spinal dumbbell tumors: an analysis of a series of 118 cases. J Neurosurg Spine 2007;7:587–593.
4. Krishnan P, Behari S, Banerji D, Mehrotra N, Chhabra DK, Jain VK. Surgical approach to C1-C2 nerve sheath tumors. Neurol India 2004;52:319–324.
5. Asazuma T, Toyama Y, Maruiwa H, Fujimura Y, Hirabayashi K. Surgical strategy for cervical dumbbell tumors based on a three-dimensional classification. Spine (Phila Pa 1976) 2004;29:E10–E14.
6. Jiang L, Lv Y, Liu XG, Ma QJ, Wei F, Dang GT, et al. Results of surgical treatment of cervical dumbbell tumors: surgical approach and development of an anatomic classification system. Spine (Phila Pa 1976) 2009;34:1307–1314.
7. Abou-Madawi AM, ElKazaz MK, Alshatoury HA, Ali SH. Far-lateral approach for ventral and ventrolateral upper cervical meningiomas: a case series and literature review. Asian Spine J 2021;15:584–595.
8. Wang J, Ou SW, Wang YJ, Wu AH, Wu PF, Wang YB. Microsurgical management of dumbbell C1 and C2 schwannomas via the far lateral approach. J Clin Neurosci 2011;18:241–246.
9. Lot G, George B. Cervical neuromas with extradural components: surgical management in a series of 57 patients. Neurosurgery 1997 41:813–820. discussion 820.
10. Liu T, Liu H, Zhang JN, Zhu T. Surgical strategy for spinal dumbbell tumors: a new classification and surgical outcomes. Spine (Phila Pa 1976) 2017;42:E748–E754.
11. George B, Dematons C, Cophignon J. Lateral approach to the anterior portion of the foramen magnum. Application to surgical removal of 14 benign tumors: technical note. Surg Neurol 1988;29:484–490.
12. Salas E, Sekhar LN, Ziyal IM, Caputy AJ, Wright DC. Variations of the extreme-lateral craniocervical approach: anatomical study and clinical analysis of 69 patients. J Neurosurg 1999;90(2 Suppl):206–219.
13. Aboul-Enein HA, Khidr WM, Abdeen KM, Madawi AA. Surgical management of ventrally based lower cervical (subaxial) meningiomas through the lateral approach: report on 16 cases. Clin Neurol Neurosurg 2015;139:152–158.
14. Azimi P, Mohammadi HR, Montazeri A. An outcome measure of functionality and pain in patients with lumbar disc herniation: a validation study of the Japanese Orthopedic Association (JOA) score. J Orthop Sci 2012;17:341–345.
15. Tomii M, Itoh Y, Numazawa S, Watanabe K. Surgical consideration of cervical dumbbell tumors. Acta Neurochir (Wien) 2013;155:1907–1910.
16. Gu BS, Park JH, Roh SW, Jeon SR, Jang JW, Hyun SJ, et al. Surgical strategies for removal of intra- and extraforaminal dumbbell-shaped schwannomas in the subaxial cervical spine. Eur Spine J 2015;24:2114–2118.
17. McCormick PC. Surgical management of dumbbell tumors of the cervical spine. Neurosurgery 1996;38:294–300.
18. Kim CH, Chung CK. Surgical outcome of a posterior approach for large ventral intradural extramedullary spinal cord tumors. Spine (Phila Pa 1976) 2011;36:E531–E537.
20. Schaller B. Spinal meningioma: relationship between histological subtypes and surgical outcome. J Neurooncol 2005;75:157–161.
21. George B, Lot G, Boissonnet H. Meningioma of the foramen magnum: a series of 40 cases. Surg Neurol 1997;47:371–379.
22. Komotar RJ, Zacharia BE, McGovern RA, Sisti MB, Bruce JN, D’Ambrosio AL. Approaches to anterior and anterolateral foramen magnum lesions: A critical review. J Craniovertebr Junction Spine 2010;1:86–99.
23. Chang HS. Posterior paramedian approach to ventrally located spinal meningioma. World Neurosurg 2017;105:755–759.
24. Joaquim AF, Almeida JP, Dos Santos MJ, Ghizoni E, de Oliveira E, Tedeschi H. Surgical management of intradural extramedullary tumors located anteriorly to the spinal cord. J Clin Neurosci 2012;19:1150–1153.
25. Slin’ko EI, Al-Qashqish II. Intradural ventral and ventrolateral tumors of the spinal cord: surgical treatment and results. Neurosurg Focus 2004;17:ECP2.
26. Watanabe K, Kakeda S, Yamamoto J, Ide S, Ohnari N, Nishizawa S, et al. Prediction of hard meningiomas: quantitative evaluation based on the magnetic resonance signal intensity. Acta Radiol 2016;57:333–340.
27. Yao A, Pain M, Balchandani P, Shrivastava RK. Can MRI predict meningioma consistency?: a correlation with tumor pathology and systematic review. Neurosurg Rev 2018;41:745–753.
28. Mayercik V, Ma M, Holdsworth S, Heit J, Iv M. Arterial spin-labeling MRI identifies hypervascular meningiomas. AJR Am J Roentgenol 2019;213:1124–1128.
29. Margalit NS, Lesser JB, Singer M, Sen C. Lateral approach to anterolateral tumors at the foramen magnum: factors determining surgical procedure. Neurosurgery 2005 56(2 Suppl):324–336. discussion 324.
|
|
|||||||||||||||||||||||||||||||||||||||