AbstractObjectiveRobotic guidance provides indirect visualization of key anatomic landmarks to facilitate minimally invasive surgery (MIS) and is emerging as a reliable and accurate technique for posterior spine instrumentation. We sought to describe eight years of experience with robotic guidance at a high-volume, multi-surgeon center. We hypothesize that robotic guidance will lead to (1) low rates of complication, readmissions, and revision surgery, (2) reduced fluoroscopic radiation exposure, (3) and accurate thoracolumbar instrumentation.
MethodsA retrospective review of complications, revision surgery, and readmission rates in patients undergoing thoracolumbar fusion surgery utilizing three robotic generations. Secondary analysis was conducted comparing the three robotic generations for complications, revision surgery, accuracy, and readmission rates along with intraoperative fluoroscopic duration.
ResultsA total of 628 patients (3,874 robotic-guided screws) ages 12–81 years-old (43.9% male) were included in the study. At one year, the cumulative complication incidence was 15.5% with a 10.3% incidence of surgical complications (3.7% wound, 1.2% robot-related, and 5.4% non-robot-related complications). At one year, the revision surgery incidence was 9.4%. There was no statistical difference between complications, readmission, or revision surgery after initial admission among the three robotic generations. The average intraoperative fluoroscopic duration was 53.8 seconds (11.9 seconds per screw and 17.6 seconds per instrumented level).
INTRODUCTIONSince the introduction of the first surgical robotic system over twenty years ago, robotic-guided surgery has become an integral tool in multiple surgical fields [1,2]. In 2004, Mazor SpineAssist® (Mazor Robotics Ltd., Caesarea, Israel) became the first Food and Drug Administration (FDA) approved robotic-guided system in spine surgery [3]. During spine surgery, robotic guidance systems, fluoroscopy, and navigation provide indirect visualization of key anatomic landmarks and facilitate minimally invasive surgery (MIS) [3-5]. The significant benefits associated with MIS have driven considerable research and development with significant advancement between robotic generations and robotic systems [3,4].
The currently available guidance systems in spine surgery can be classified into three general categories: standard navigation-based systems with optical tracking reference markers (NAV), robotic arms combined with a NAV system (RNAV), and anatomy recognition-based robotic systems. RNAV systems utilize optical reference markers attached to the patient and a floor-mounted robotic arm registered with intraoperative 3-dimensional (3D) imaging; anatomy recognition-based robotic systems utilize bed- or patient-mounted systems fixed to bony landmarks and preoperative planning from 3D imaging. Mazor CoreTM technology (MCT) (Medtronic, Minneapolis, MN, USA) utilizes automated anatomy recognition software that registers individual vertebrae on two-view intraoperative fluoroscopic imaging with preoperative 3D images and determines the patient’s intraoperative location relative to the robotic system.
The current literature suggests that robotic guidance systems can be used to perform reliable and accurate thoracolumbar pedicle instrumentation [5-8]. However, significant variation between robotic technology over time and individual proprietary robotics systems introduces significant heterogeneity in the body of research. Findings related to accuracy or safety of one robotic guidance system cannot be directly attributed to other robotic guidance systems, and as such, independent studies of each system are ultimately necessary. The current study reviews the experience across three robotics systems over eight years utilizing MCT for thoracolumbar fusion surgery at a single high-volume, multi-surgeon center. We hypothesize that robotic guidance will lead to low rates of complication and revision surgery, reduced fluoroscopic radiation exposure, and accurate thoracolumbar instrumentation.
MATERIALS AND METHODSThis study was approved by the Institutional Review Board of Advarra (IRB No. Pro00034175).
1. Study Design and Patient SelectionWe conducted a retrospective review of robotic-guided thoracolumbar spine surgeries at a multi-surgeon, single center between July 2012 and March 2020. The experience included three robotic generations using MCT evolving over time. Renaissance® (R), Mazor X® (X), and Mazor X Stealth Edition® (MXSE) systems were utilized for robotic guidance (Medtronic, Minneapolis, MN, USA) as shown in Figure 1. R was implemented in our practice in 2012, X in 2018, and MXSE in 2019.
All consecutive adolescent and adult patients undergoing robotic-guided thoracolumbar fusions for deformity or degenerative spine conditions were included in the study. These included both primary and revision surgeries, as well as MIS and open approaches. All patients or their legal guardians signed written informed consent within the institute’s Notice of Privacy Practices prior to surgery. IRB approval was granted by Advarra, a centralized IRB (Pro00034175).
2. Data CollectionData including short- and long-term complications, revision surgery, and intraoperative fluoroscopic exposure were retrospectively collected from medical records and operative reports. Complications were subdivided into the following time periods: intraoperative, initial hospitalization, and postoperative at 30-days, 90-days, and 1-year. Intraoperative records were reviewed for the following: dural tear, estimated blood loss (EBL), neurologic deficit, other complications, and misplaced screws. For the purposes of this study, ‘misplaced’ was defined as any screw that required intraoperative revision of trajectory or removal as a result of pedicle screw stimulation less than 10 mA or if a breach was identified on intraoperative 3D imaging when available. Intraoperative fluoroscopic exposure was measured by intraoperative fluoroscopic duration in seconds and reported as total radiation duration, time per instrumented level, and time per robotic-executed screw. Postoperative complications were divided into surgical and medical complications. Surgical complications were subdivided into wound (including infection), robot-related (including misplaced screws and implant-related durotomy), and non-robot-related complications. Revision surgeries were reviewed at the initial admission, 30-days, 90-days, and 1-year. Medical complications occurring after 90 days postoperatively were deemed unlikely related to the robotic-guided portion of surgery and were not included.
Patient demographics, Charlson comorbidity index (CCI), body mass index (BMI), nicotine status, and primary preoperative diagnosis were collected from medical records and operative reports. Perioperative variables including EBL, number of screws, types of screws, procedure time, number of instrumented levels, length of hospital stay (LOS), and readmissions were also recorded. Postoperative computed tomography (CT) scans were available for 184 cases (a total of 1,255 screws). An independent, board-certified neuroradiologist reviewed and graded these screws for accuracy using the Gertzbein-Robbins (GR) classification [9].
3. StatisticsDescriptive statistics and cohort comparison analysis were performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA). Fisher’s exact test and chi-squared test were used to compare variables across robotic cohorts. A p-value threshold of 0.05 was used to determine statistical significance.
RESULTSThis study included a total of 3,874 robotic-guided screws in 628 patients ages 12–81 years-old (mean 51.8±13.7 years) who were 43.9% male. Patients had an average BMI of 29.8 kg/m2, CCI score of 1.2, and 7.8% were nicotine users (Table 1). R was used in 33.4% of the total cases, X in 41.1% and MXSE in 25.5%. The case majority was primary fusions (88.5%) and staged anterior/posterior procedures (88.9%). The most common surgical indication, spondylolisthesis, accounted for 46.2% of all cases (Table 2). Of patients, 64.2% had at least 1 year of postoperative follow up with an average follow up duration of 18.0±16.3 months (Table 1).
1. Complications, Revision Surgery, and Readmissions1) Initial AdmissionA total of 9 patients (1.4%) experienced an intraoperative complication, including 8 durotomies unrelated to instrumentation and 1 episode of bradycardia. Of the 3,874 screws placed with robotic guidance, 46 (1.2%) were considered misplaced resulting in an initial robotic-guided screw placement accuracy of 98.7% (Table 3).
A total of 33 patients (5.3%) experienced a complication following surgery, during the initial admission (Table 3). Twenty-one patients (3.3%) experienced a surgical complication including: 2 hematomas (0.3%) (counted as wound complications), 6 robot-related (1.0%), and 13 non-robot-related complications (2.1%). The robot-related surgical complications were symptomatic screws identified on postoperative imaging to be malpositioned and required revision surgery during initial admission. After revision surgery, 100% achieved symptom resolution (Table 3).
Twelve patients (1.9%) required a revision surgery during the initial admission including: the 6 symptomatic malpositioned screws, 2 retroperitoneal hematomas requiring evacuation, 3 patients with radiculopathy unrelated to the instrumentation requiring posterior decompression, and 1 anterior migration of interbody implant after posterior instrumentation, requiring revision (Table 3).
2) 30 DaysAt 30-days the cumulative number of patients who experienced a complication was 68 (10.8%). Cumulative surgical complications at 30 days included 14 wound (2.2%), 7 robot-related (1.1%), and 18 non-robot-related complications (2.9%). These included the following new wound complications since admission: 7 wound infections, 2 seromas, 2 retroperitoneal hematomas, and 1 epidural hematoma. One patient underwent a postoperative CT scan due to non-robot-related distal construct fracture and a screw breach was noted proximally within the construct incidentally, which was asymptomatic. This was counted as the 1 new robot-related complication because the misplaced screw was removed during the revision surgery to extend the construct distally (Table 3).
The 30-day cumulative revision surgery rate was 4.8% including: 12 wound-related incision and drainage (I&D), 1 incision and drainage for arterial line site infection, 4 decompressions, 1 revision PIF, and 1 PIF extended proximally for junctional failure. The 30-day cumulative readmission rate was 3.8%, including six medical readmissions and eighteen for revision surgery (Table 3).
3) 90 DaysThe 90-day cumulative complication incidence was 13.5% (Table 3). Cumulative surgical complications included 4.0% wound, 1.1% robot-related, and 3.8% non-robot-related complications. In the 90-day period new wound complications included: 10 wound infection and 1 epidural hematoma. No new robot-related complication occurred during the 90-day follow up period (Table 3).
Eighteen patients underwent revision surgery between the 30- and 90-day marks, resulting in a 7.5% cumulative revision surgery rate. The 90-day revision surgeries included: 11 wound-related I&Ds, 5 additional decompressions, 1 ALIF revision, and 1 PIF extended distally for distal junctional failure. The cumulative readmission rate at 90 days was 6.5%. Twenty new readmissions occurred between the 30- and 90-day period, 18 for revision surgery and 2 for medical complications (Table 3).
4) 1 YearA total of 406 patients reached a 1-year follow-up. The cumulative complication incidence for these patients was 15.5%. Surgical complications were 3.7% wound, 1.2% robot-related, and 5.4% non-robot-related cumulative complications. Between 90 days and 1 year follow-up, 10 new complications occurred that were not robot-related. These included one wound infection, five patients with radiculopathy, and four patients with proximal junctional kyphosis. All ten patients underwent revision surgery resulting in a cumulative revision surgery incidence of 9.4% (Table 4).
2. Robotic Cohort ComparisonOver time, patients undergoing robotic-guided surgery were older with a higher CCI score, a greater number of instrumented levels, and more likely to require an open procedure versus MIS (Table 1, 2). As a result, total procedure time, net robot time, and number of robotic-guided screws also increased over time. There was no statistical difference between cumulative complications, readmission, or revision surgery after initial admission among the three robotic generations. MXSE cohort required no revision surgeries during the initial admission compared with 3.5% in the X cohort and 1.4% in R (p=0.033) (Table 3, 4).
3. Fluoroscopic Radiation ExposureThe average total fluoroscopic duration was 53.8 seconds. The average fluoroscopic duration per screw was 11.9 seconds per screw and 17.6 seconds per instrumented level. The mean fluoroscopic duration in the MXSE cohort was 41.0 seconds, the lowest across the three generations (vs. X: 55.3 seconds and R: 61.6 seconds, p<0.001). Over time, the evolving robotic guidance systems led to less intraoperative radiation. A statistically significant decrease in intraoperative radiation was found in the MXSE cohort compared to the X and R cohorts (Table 2).
4. AccuracyOf the 3,874 screws, 1,255 screws had postoperative computed tomography (CT) scans available for independent review. Of the graded screws, 1,153 screws (91.9%) were classified as Gertzbein-Robbins (GR) grade A or B. There was no statistically significant difference in GR grading between the three robotic cohorts (Table 5).
DISCUSSIONInnovation in robotic guidance has been driven by a desire to improve patient safety and surgical efficacy. The current generations of robotic systems combine precise anatomic landmark identification with specialized surgical planning software to achieve this goal. The literature supports improved accuracy and reliability with robotic-guided systems in spine surgery with accuracy rates as high as 99% [10-12]. The current consensus from several systematic reviews and meta-analyses supports high accuracy rates and the potential benefits of robotic guidance; however, the current body of evidence is limited by substantial heterogeneity between systems and a lack of complication and revision surgery data [4-7,10,13,14]. The few studies evaluating complications and revision surgery focus exclusively on wound complications and revision surgery for malpositioned screws during the early postoperative period [11,15,16]. The authors seek to expand the scope of the current literature by reporting both short-term and long-term complications, revision surgery, and readmission rates at a high-volume spine surgery center across the R, X, and MSE robotic systems.
Our results found low cumulative complication (15.5%), revision surgery (9.4%), and 90-day readmission rates (6.5%) across all three generations of robotic systems at 1 year follow up. These results are consistent with or better than the limited literature on instrumentation-related complications and revision surgery. In our study of 628 consecutive cases and 3,874 robotic-guided screws, seven patients required revision surgery for robot-related complications, an overall rate of 1.1%. Six of the seven required revision surgery during their initial admission for implant-related radiculopathy. Siccoli et al.’s [4] recent systematic review and meta-analysis comparing robotic-guided, navigation-guided, and freehand thoracolumbar pedicle screw placement was unable to draw conclusion on complication rates with robotic guidance due to lack of sufficient data. Fourteen of the 31 studies included in the systematic review reported complication rates, but none of the fourteen were determined to be high-quality studies [4]. A more recent comparison study of 46 O-arm Navigation and 39 Mazor X patients found no difference in wound-related complication rates between the two groups [17]. Another small matched cohort study reported an 8.7% 30-day complication rate with robotic-guidance, but it does not discuss what types of complications were tracked or occurred [16].
Two recent studies evaluated perioperative outcomes between robotic guidance (RG) and fluoro-guidance (FG) in adult lumbar fusions in the prospectively collected, multicenter MIS ReFRESH database [8,18]. Good et al. [8] evaluated a total of 485 patients: 374 in the RG arm and 111 in the FG arm. Patients in the RG group had a 10.4% surgical complication rate and 2.1% rate of revision surgery at 1 year compared to 35.1% and 6.3%, respectively, in the FG arm. They found patients in the FG arm were 5.8 times more likely to develop a complication (HR=5.8, 95% CI: 3.5–9.6, p<0.001), and 11.0 times more likely to require a revision surgery (HR=11.0, 95% CI 2.9–41.2, p<0.001) compared to those in the RG [8]. Liounakos et al. [18] found reduced complications and revisions in patients undergoing robotic-guided fusions. They reported an 8.02% 90-day complication rate with short segment robotic-guided fusions and 83.2% reduction in complications compared to freehand technique (p<0.001) [18].
Lieber et al. [19], utilizing the National Inpatient Sample, matched robotic-guided and conventional short-segment lumbar fusions to compare complication rates. They found a 31.9% and 8.2% complication rate for minor and major complications, respectively in the robotic-guided group. There was an overall complication rate of 36.2% in the robotic-guided group compared to 21.0% in the conventional technique group (p<0.001); statistical significance was lost after controlling for confounding factors in their multivariate analysis [19].
Staartjes et al. [13] conducted a systematic review and meta-analysis comparing revision surgery rates between robotic-guided, navigated, and freehand techniques for thoracolumbar instrumentation and reported insufficient evidence of the superiority of robotic-guidance or navigation. Variations in complication rates reported in the current literature may be attributable to differences in both complication definitions between studies and complication data collection. These differences and limited current literature prevent comparison between studies [20-22].
This study found an average total fluoroscopic duration of 53.8 seconds (11.9 seconds per screw, 17.6 seconds per instrumented level). The current literature suggests that robotic guidance reduces intraoperative fluoroscopic duration and radiation exposure compared to conventional freehand technique [5,10,12,23]. This reduction in intraoperative radiation reduces the cumulative exposure and radiation risk to surgeons and OR staff. It is well documented that cumulative exposure to radiation in spine and orthopaedic surgery increases the risk of multiple health conditions [24-27]. Robotic-guidance relies on CT or O-arm imaging which may expose the patient to higher radiation, but the risk of this limited additional exposure must be weighed against the benefit of high-resolution imaging and added accuracy and surgical precision benefits.
In a randomized control trial comparing MIS robotic-guided and fluoroscopy-guided lumbar fusions, Hyun et al. [21] found fluoroscopic time per screw was decreased four-fold in the robotic vs. fluoroscopic group (3.5 seconds vs. 13.3 seconds, respectively). Another study by Roser et al. [28] found a similar reduction in fluoroscopic duration in robotic-guided surgery when patients were randomized into fluoroscopic-guided, navigation, or robotic-guided arms.
In the current study, 91.9% of the 1,255 robotic screws with postoperative CT scans were GR Grade A or B. In the R cohort, 92.2% of screws were GR Grade A or B (vs. 92.0% and 90.8%, in the X and MSE cohorts respectively). No statistically significant difference was seen in GR Grade between the three robotic cohorts (p=0.976). The accuracy rate found in the current study is consistent with rates reported in the current literature, ranging from 90% to 100% [5,7,10,12-14].
The retrospective observational study design results in several inherent limitations. In this multi-surgeon, single-center study, differences among surgical techniques and robotic experience between surgeons may introduce unrecognized confounding. Without a control group, the current study cannot reach conclusions comparing robotic-guided instrumentation with other techniques. The goal of this study is to present a high-volume, multi-surgeon spine institute’s experience with complication, revision surgery, and readmission rates in robotic-guided thoracolumbar instrumentation.
ACKNOWLEDGEMENTSStatistical analytic support by Dr. Leah Y. Carreon, MD, Clinical Research Director, Norton Leatherman Spine, Louisville, KY, USA.
Fig. 1.Images demonstrating the (A) Renaissance robotic system, (B) Mazor X robotic system, and (C) Mazor X Stealth Edition robotic system which includes the addition of the navigation camera and software upgrade. 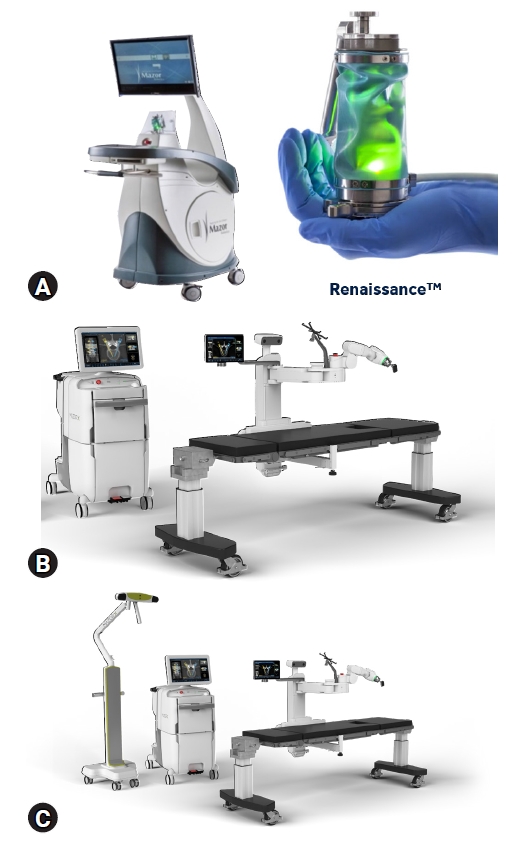
Table 1.Cohort comparison (patient demographics)
Table 2.Cohort comparison (surgical variables)
Table 3.Complication, revision surgery, and readmission comparison
Table 4.One year complication, revision surgery, and readmission comparison Table 5.Accuracy comparison using Gertzbein and Robbins (GR) grade REFERENCES1. Dogangil G, Davies BL, Rodriguez y Baena F. A review of medical robotics for minimally invasive soft tissue surgery. Proc Inst Mech Eng H 2010;224:653–679.
2. Alkatout I, Mettler L, Maass N, Ackermann J. Robotic surgery in gynecology. J Turk Ger Gynecol Assoc 2016;17:224–232.
3. D’Souza M, Gendreau J, Feng A, Kim LH, Ho AL, Veeravagu A. Robotic-assisted spine surgery: history, efficacy, cost, and future trends. Robot Surg 2019;6:9–23.
4. Siccoli A, Klukowska AM, Schröder ML, Staartjes VE. A systematic review and meta-analysis of perioperative parameters in robot-guided, navigated, and freehand thoracolumbar pedicle screw instrumentation. World Neurosurg 2019;127:576–587.e5.
5. Fiani B, Quadri SA, Farooqui M, Cathel A, Berman B, Noel J, et al. Impact of robot-assisted spine surgery on health care quality and neurosurgical economics: a systemic review. Neurosurg Rev 2020;43:17–25.
6. Ghasem A, Sharma A, Greif DN, Alam M, Maaieh MA. The arrival of robotics in spine surgery: a review of the literature. Spine (Phila Pa 1976) 2018;43:1670–1677.
7. Joseph JR, Smith BW, Liu X, Park P. Current applications of robotics in spine surgery: a systematic review of the literature. Neurosurg Focus 2017;42:E2.
8. Good CR, Orosz L, Schroerlucke SR, Cannestra A, Lim JY, Hsu VW, et al. Complications and revision rates in minimally invasive robotic-guided versus fluoroscopic-guided spinal fusions: the MIS ReFRESH prospective comparative study. Spine (Phila Pa 1976) 2021;46:1661–1668.
9. Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976) 1990;15:11–14.
10. Li HM, Zhang RJ, Shen CL. Accuracy of pedicle screw placement and clinical outcomes of robot-assisted technique versus conventional freehand technique in spine surgery from nine randomized controlled trials: a meta-analysis. Spine (Phila Pa 1976) 2020;45:E111–E119.
11. Benech CA, Perez R, Benech F, Greeley SL, Crawford N, Ledonio C. Navigated robotic assistance results in improved screw accuracy and positive clinical outcomes: an evaluation of the first 54 cases. J Robot Surg 2020;14:431–437.
12. Gao S, Lv Z, Fang H. Robot-assisted and conventional freehand pedicle screw placement: a systematic review and meta-analysis of randomized controlled trials. Eur Spine J 2018;27:921–930.
13. Staartjes VE, Klukowska AM, Schröder ML. Pedicle screw revision in robot-guided, navigated, and freehand thoracolumbar instrumentation: a systematic review and meta-analysis. World Neurosurg 2018;116:433–443.e8.
14. Yu L, Chen X, Margalit A, Peng H, Qiu G, Qian W. Robot-assisted vs freehand pedicle screw fixation in spine surgery - a systematic review and a meta-analysis of comparative studies. Int J Med Robot 2018;14:e1892.
15. Schröder ML, Staartjes VE. Revisions for screw malposition and clinical outcomes after robot-guided lumbar fusion for spondylolisthesis. Neurosurg Focus 2017;42:E12.
16. Jiang B, Pennington Z, Azad T, Liu A, Ahmed AK, Zygourakis CC, et al. Robot-assisted versus freehand instrumentation in short-segment lumbar fusion: experience with real-time image-guided spinal robot. World Neurosurg 2020;136:e635–e645.
17. Mao G, Gigliotti MJ, Myers D, Yu A, Whiting D. Single-surgeon direct comparison of O-arm neuronavigation versus Mazor X robotic-guided posterior spinal instrumentation. World Neurosurg 2020;137:e278–e285.
18. Liounakos JI, Kumar V, Jamshidi A, Silman Z, Good CR, Schroerlucke SR, et al. Reduction in complication and revision rates for robotic-guided short-segment lumbar fusion surgery: results of a prospective, multi-center study. J Robot Surg 2021;15:793–802.
19. Lieber AM, Kirchner GJ, Kerbel YE, Khalsa AS. Robotic-assisted pedicle screw placement fails to reduce overall postoperative complications in fusion surgery. Spine J 2019;19:212–217.
20. Kim HJ, Jung WI, Chang BS, Lee CK, Kang KT, Yeom JS. A prospective, randomized, controlled trial of robot-assisted vs freehand pedicle screw fixation in spine surgery. Int J Med Robot 2017;13:e1779.
21. Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine (Phila Pa 1976) 2017;42:353–358.
22. Kantelhardt SR, Martinez R, Baerwinkel S, Burger R, Giese A, Rohde V. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J 2011;20:860–868.
23. Peng YN, Tsai LC, Hsu HC, Kao CH. Accuracy of robot-assisted versus conventional freehand pedicle screw placement in spine surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Transl Med 2020;8:824.
24. Dewey P, Incoll I. Evaluation of thyroid shields for reduction of radiation exposure to orthopaedic surgeons. Aust N Z J Surg 1998;68:635–636.
25. Cantone MC, Ginjaume M, Miljanic S, Martin CJ, Akahane K, Mpete L, et al. Report of IRPA task group on the impact of the eye lens dose limits. J Radiol Prot 2017;37:527–550.
26. Mastrangelo G, Fedeli U, Fadda E, Giovanazzi A, Scoizzato L, Saia B. Increased cancer risk among surgeons in an orthopaedic hospital. Occup Med (Lond) 2005;55:498–500.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||