AbstractObjectiveOblique anterior to psoas (ATP) interbody lumbar fusion is associated with advantages such as sufficient indirect decompression and restoration of lordosis. Therefore, a comprehensive preoperative assessment that includes the location of entry into the disc space, a feasible trajectory to complete the intervertebral space procedure, and the possible retraction of the psoas muscle is necessary to correctly and safely perform the technique.
MethodsFrom January 2019 to January 2020, 160 lumbar CT scans were evaluated. Only 124 images from the L2-L3, L3-L4, and L4-L5 levels met the inclusion criteria. The length of the anterior vertebral line (AVL) and the middle-third of the disc in the anteroposterior axis were measured to localize the entry point (EP). The distance between the anterior arterial vessel (AV) and the EP was also measured. The trajectory commonly used to set the surgical instruments into the disc space was called α, and a new proposed trajectory termed β was calculated. The psoas cross-sectional area anterior to the β angle trajectory was measured to determine any possible retraction using this parameter.
ResultsThe EP-AVL distances were L2-L3 11.49 ± 0.89 mm, L3-L4 11.54 ± 0.88 mm, and L4-L5 11.57 ± 0.87 mm. The EP-AV lengths were 17.64 ± 5.62 mm, 19.36 ± 5.49 mm, and 16.48 ± 6.47 mm at L2-L3, L3-L4, and L4-L5, respectively. The average α and β trajectory angles reported were 39.91º and 14.48º, respectively. Psoas muscle retraction was primarily noted at the L4-L5 level.
INTRODUCTIONSince Mayer [1] described the retroperitoneal anterior to psoas (ATP) microsurgical approach for the interbody fusion of L2 to L5 in 1997, and Silvestre et al. [2] reported the first retrospective outcomes of 179 patients who underwent the same approach in 2012, the oblique ATP lumbar interbody fusion has been adopted by spine surgeons worldwide.
The procedure-related advantages over the transpsoas technique are the lower incidence of lumbar plexus injury and the minor retraction of the psoas muscle leading to a lower postoperative neuromuscular deficit in the thigh [3-6].
The primary concern is the intricate anatomy of the surgical corridor available to address the degenerated disc in specific cases. The oblique surgical entry is limited by the left lateral border of the aorta or left iliac artery and the anterior medial border of the psoas muscle [7]. Variations in the location of these structures can obstruct or modify the surgical corridor to reach the disc, thereby increasing the risk of fatal intraoperative vascular complications [8]. Therefore, anatomical research based on different imaging modalities and cadaveric studies has detailed the features of the left-sided ATP oblique corridor and the surrounding spinal retroperitoneal structures [7-17].
A critical appraisal that has not been addressed yet in the medical literature is how to plan a safer trajectory of the oblique ATP approach that reduces the risk of injuring contralateral anatomical structures or avoids breaking into the contralateral foramen due to the oblique nature of the approach.
The oblique ATP lumbar fusion technique execution includes the well-known orthogonal maneuvering, which consists of positioning the instruments as perpendicular as possible to the disc when working within the intervertebral space. However, the more dorsal retraction of the psoas muscle when instruments levering, the potential injury of the lumbar plexus is possible. The contralateral neural structures are at risk of being transgressed during the whole process of intervertebral space preparation to deliver the cage because an oblique trajectory is followed.
For these reasons, preoperative planning of a trajectory to execute the whole oblique ATP technique with the lower risk of transgressing contralateral nerve structures and simultaneously knowing the possible transverse area of the psoas muscle that will be retracted with the selected trajectory is necessary. It requires identifying an entry point to the disc and measuring the different angles to approach disc space.
This article suggests an imaging-based routine preoperative assessment of patients who will undergo an oblique ATP lumbar fusion and also analyzes and discusses the results obtained through a preclinical morphometric CT-based anatomical study in an American Hispanic population.
MATERIALS AND METHODSThe ethics committee of Hospital H+ Queretaro approved this study (no.10.21JQO). From January 2019 to January 2020, 160 computed tomography (CT) images of the lumbar spine obtained randomly from the Hospital’s Radiology Department were measured. The scanner's parameters of non-enhanced CT images were: slice thickness of 5 mm, pitch of 1.15 mm, and reconstructive slice thickness of 1 mm. Lumbar CT scans were excluded from patients with a history of previous lumbar or retroperitoneal surgery, vertebral malformation, spinal deformity, infection, fractures, and spinal tumors. Also, lumbar levels with high-rising psoas (Mickey Mouse ear-like) and less than 5 mm between the left lateral border of the aorta or left iliac artery and the anterior ventral medial border of the psoas muscle were excluded. Two different researchers meticulously reviewed CT images at different times. Demographic data (i.e., sex, age, and BMI) were recorded for all included CT scans.
1. Preoperative AssessmentThe following parameters were planned and measured in the axial views at the midpoint disc height of the L2-L3, L3-L4, and L4-L5 segments. 1) The disc contour was divided into thirds from anterior to posterior. The middle one was considered the ideal location to place the interbody device using an oblique trajectory (Figure 1A). 2) The entry point (EP) to the disc was planned in the upper left corner of the middle third. The distance between the EP and the anterior vertebral line was measured (Figure 1B). 3) The distance between the left lateral border of the aorta or iliac artery (anterior vessels) and the EP was measured (Figure 1C). 4) Two different oblique trajectories to approach the intervertebral disc were simulated; the α and β trajectories. The α is tangential to the anterior border of the psoas muscle (Figure 1D), and the β crosses the psoas muscle and intersects the EP and the inferior right corner of the middle third (Figure 1E). Both trajectories meet the coronal line forming an angle measured. The cross-section area of the whole psoas muscle and the psoas ventral to the β trajectory was defined. Then, the percentage of psoas retraction was calculated as follows: the cross-sectional area of the psoas ventral to the β trajectory (100)/the cross-sectional area of the whole psoas. Finally, an example of a cage inserted at L3-L4 under these parameters is shown in Figure 1F.
2. Statistical AnalysisThe distances between the anterior vertebral line and anterior vessel to the EP were expressed as mean±standard deviation (SD) with minimum and maximum in mm. The α and β trajectories were reported as mean±SD with minimum and maximum degrees. The different measures of the psoas cross-sectional area were reported in mm2, with the percentage retracted. An unpaired t-test was used to analyze the differences in the distances with the EP between sexes, with p≤0.05 being statistically significant. One-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test was used to analyze differences in the distances mentioned and the possible retracted psoas cross-sectional area between levels with p<0.05 indicating statistical significance. IBM SPSS version 24 software (IBM Corp., Armonk, NY, USA) was employed for the statistical analysis.
RESULTSA total of 160 non-enhanced lumbar CT scans were revised. Of these, 36 CTs did not meet the inclusion criteria (16.9% did not have a left-sided oblique corridor, 4.4% had high-rising psoas, and 6.2% had other exclusion criteria, e.g., deformity, retroperitoneal surgery, previous spinal surgery). In the present study, 124 CT scans met inclusion criteria. CT scans from 67 male and 57 female patients with a mean age of 47.9±15.3 (min, 18; max, 82) years and a mean BMI of 25.27±3.02 (min, 18.48; max, 34.50) were included in the study. Measurements at the L2-L3, L3-L4, and L4-L5 levels were completed for all 124 CT scans.
The distance between the EP to the anterior vertebral line was 11.49±0.89 mm at L2-L3, 11.54±0.88 mm at L3-L4, and 11.57±0.87 mm at L4-L5. A shorter distance was found in females at all levels than males (Table 1). The mean distance between the EP to the anterior vessel (AV) was 17.64±5.62 mm at L2-L3, 19.36±5.49 mm at L3-L4, and 16.48±6.47 mm at L4-L5. The only statistical difference in EP-AV distance was found at the L4-L5 compared to the other levels (p<0.001). A comparison of EP-AV distances by sex revealed that females had a significantly smaller mean value at both the L3-4 (17.44±5.10 vs. 20.99±5.30; p<0.001) and L4-5 (15.44±5.89 vs. 17.35±5.83; p=0.007) levels as compared to males. Women had shorter EP-AV distances than men. The most considerable EP-AV distance was observed at the L3-L4 level, while the smallest was L4-L5 (Figure 2).
At the L2-L3, L3-L4, and L4-L5 levels, the mean angle of α trajectory was 38.16°±11.37°, 39.88°±9.66°, and 41.68°±10.15°, respectively. Men had steeper angle values than women, and the L4-L5 level had the steepest angle of the α trajectory in both sexes (Table 2). The comparison of α trajectory angles between levels showed a statistically significant difference at L4-L5 (p=0.008), but comparison by sex did not reveal any differences (p>0.05) (Table 2). The mean angle of β trajectory at L2-L3, L3-L4, and L4-L5 were 14.86°±3.55°, 14.40°±2.43°, and 14.17°±1.97°, respectively. These angles were shallower than the α trajectory angles (Table 2). A comparison of the β trajectory angles between levels revealed a significant difference at the L4-L5 compared with the other levels (p=0.004). No significant difference was observed between the sexes (Table 2). L4-L5 had the shallowest β trajectory angle in both sexes. The mean psoas cross-sectional area retracted at each level using the β trajectory was 120.32 mm2 at L2-L3, 250.13 mm2 at L3-L4, and 399.63 mm2 at L4-L5 (Table 3). The comparison between levels was statistically significant (all p<0.05), and the percentage of the psoas retracted is shown in Figure 3.
DISCUSSIONSuccessful indirect decompression placing a larger interbody cage is one of the procedure-related advantages of lateral and anterolateral lumbar fusion techniques [18-20]. Another benefit is the approach’s ability to restore lordosis compared with other fusion techniques [6,21]. In addition, other factors contribute to adequately restoring sagittal balance. Such factors include the cage type (neutral or lordotic cage), the position of the implant within the intervertebral space, the more anterior, the more lordosis, and maneuvers to release the lumbar spine. Nonetheless, with more anterior surgical corridors, there is a higher risk of vascular injury and subsidence [22]. Alternatively, while a more posterior cage diminishes sagittal balance restoration, it also lessens the risk of subsidence. It must also be noted that with a more posterior approach, a slightly higher risk of damage to the psoas and lumbar plexus due to its dorsal location can occur, especially at L4-L5 [22,23].
The surgical corridor immediately anterior to the center of the intervertebral space in the anteroposterior direction has been considered convenient for placing the interbody device in lateral techniques. However, it depends on the lumbar plexus location at levels [22,24].
The oblique ATP approach has gained popularity among spine surgeons interested in minimally invasive techniques in recent years. This procedure shares the advantages of the transpsoas approach, including reduced blood loss, improved postoperative pain, faster recovery, and preservation of posterior muscle and ligament structures. The most notable procedure-related advantage of the oblique ATP approach is the minor violation of the lumbar plexus because the surgical corridor is anterior to the psoas muscle [6]. Several authors reported an incidence of 5% to 14% for postoperative neuromuscular symptoms in the thigh after oblique lumbar interbody fusion, compared to the 19% to 33% seen in patients who underwent the transpsoas technique [25-28]. However, the primary concern among surgeons interested in oblique fusion is the risk of abdominal arterial injury. Interestingly, this technique's arterial injury rates are low and previously reported as 0.3% to 2.4% [29].
To make the procedure as safe as possible, several image-based and cadaveric dissection studies have intensively examined the surgical ATP corridor [7-17]. Most of these publications are based on Asian [8-14,16] and American [7,15,17] populations. Therefore, while the general considerations have been well described (e.g., the anatomical elements involved in the oblique surgical corridor and the elements surrounding the lumbar spine at each level), data on the North American/American Hispanic population are lacking.
The following concerns are addressed in the present article: 1) How could a safe entry point (EP) to the intervertebral space (IVS) be planned for an oblique ATP approach? 2) How to plan a safe oblique trajectory to the IVS for the contralateral neural elements and vessels anterior to the spine? And finally, 3) How is the potential retraction of the psoas muscle calculated preoperatively using the parameters proposed?
Three points must be considered before an oblique lumbar fusion. 1) An oblique surgical corridor from 0.5 to 10 mm, limited by the left lateral border of the anterior vessel (aorta or left iliac artery) and the anterior belly of the psoas. 2) The surgeon should opt for other fusion techniques in patients with high-rising psoas due to the risk of excessive manipulation. 3) Assess the location of the anterior vessel relative to the disc space to avoid arterial injury.
Wang et al. [9] studied the anterior vessel and psoas muscle locations relative to the disc space. The authors constructed different models to specifically study the prevalence of any one scenario. The most prevalent models were to locate the vessel distal to the psoas at L2-L3 and L3-L4, enlarging the surgical corridor, and the psoas closer to the anterior vessel at L4-L5, shrinking the corridor. Similar to the oblique corridor length reported by Davis et al. [7] in a cadaveric study of Americans. They found L4-L5 to be the narrowest with a 15.00 mm distance, L2-L3 18.60 mm, and L3-L4 19.25 mm.
Here we propose an oblique ATP approach where the entry point is not based on the psoas muscle location due to its anatomical variability at each lumbar level. The patient must have a feasible surgical corridor: a distance from the proposed entry point to the anterior vessel at least more than 5 mm. In our case series, the entry point proposed was located, on average, 11.53 mm away from the anterior vertebral line (AVL) in the lumbar levels measured (L2-L5). To calculate the entry point to AVL distance as part of this preoperative assessment proposed in the present study would allow starting the oblique approach far from the at-risk zone located anterior to the spine. It would enable the surgeon to start the oblique ATP approach with a pre-planned target to place the initial needle at one particular point (entry point) on the IVS based on the AVL. The average distance between the anterior vessel and entry point was 17.83 mm and was longer in men (19.05 mm) than in women (16.38 mm). It means that the routine prior recognition of this information will provide safety to the approach since the entry point is far from the anterior vessels of the spine.
Our findings are similar to other studies based on different populations. Our results confirmed that the L4-L5 level has the narrowest entry point to anterior vessel distance in Hispanics, suggesting that ethnicity is not a determinant in the entry point to anterior vessel measure [7-17].
The oblique trajectory to the IVS could injure contralateral neural structures mainly if the angulation is not meticulously planned or if a more significant than required interbody cage is selected. The more posterior the trajectory or steeper the angled trajectory, the greater the risk of damage to the contralateral lumbar plexus, exiting nerve root, and thecal sac. The surgeon must also consider the orthogonal maneuver while tapping the cage to set it into the prepared disc space. A steeper angle means a prolonged orthogonal maneuver and a greater compression risk for the ipsilateral psoas muscle and lumbar plexus.
An MRI-based imaging study within the Asian population evaluated different scenarios using simulated trajectories from 0º to 45º [16]. They found that a 15º trajectory for placing the interbody device is associated with a lower risk of damaging contralateral neural structures [16]. In our study, the trajectory called α depended on the location of the anterior edge of the psoas; the surgeon usually chooses this to set the cage in a real-life scenario. The average of the α trajectory angle was 39.9º, which is dangerous for the reasons mentioned. Our results also indicated that the steepest trajectory was observed at L4-L5 in both sexes. This can be attributed to the most significant volume of the psoas being found in the caudal rather than lumbar cranial levels.
The angle of β trajectory we found in Hispanics was 14.48º on average. The β trajectory was determined based on setting the interbody device in the middle-third of the intervertebral space; therefore, it can be planned before the surgery. This parameter is relevant because not all medical centers are equipped with intraoperative navigation technologies. Furthermore, our study confirmed that the trajectory reported by Huang et al. [16] is safest using anatomical measurements. We inferred that there was no significant difference in the angle of β trajectory values among the lumbar levels since it depends on the size of the middle-third region and the anteroposterior lengths of the lumbar vertebral bodies were similar.
Finally, we analyzed the approximate percentage of psoas retraction during the interbody cage placement following the β trajectory. The results revealed that between 20% to 30% of the psoas cross-sectional area anterior to the β trajectory could be manipulated temporarily during cage insertion. This indicates that oblique lumbar fusion is not free of psoas manipulation, and the surgeon should keep it in mind when opting for this procedure.
LimitationsAn age-based subgroup would have enabled determining the influence of age on the β trajectory. Only two experienced researchers did the measurements. An image-based study could expose differences with accurate anatomical findings. Our study used non-enhanced lumbar CT scans in a supine position, which may differ from right/left lateral decubitus position. No CT scans from patients with deformities were included, which may affect the parameters. This article is an observational retrospective morphometric preclinical research study based on a particular population at a single institution and limits its generalizability. A North American/American Hispanic population from Mexico and similarities or differences among all Hispanics (i.e., American, South American, and Iberic) should be considered in future studies.
CONCLUSIONOur study proposes an entry point that provides access to the disc space laterally in the middle-third and away from the anterior arterial lumbar vessels that depend on the anterior vertebral line. It could permit the introduction of the surgical instruments, prepare the intervertebral space and obliquely place the cage with a different trajectory proposed and termed β, with which it is possible to measure the potential psoas muscle retracted during the procedure. The parameters presented and analyzed in this research could serve as a preoperative assessment in Hispanic North American/American patients recommended for an oblique ATP lumbar fusion.
Figure 1.Proposed CT-based preoperative assessment for planning an oblique trajectory. (A) The middle third is shown in red. (B) Distance between the anterior vertebral line (white line) and the entry point. (C) Distance between the anterior vessel (AV) and the entry point (green dotted line). (D) The α and (E) β trajectories. The white arrow pointed to the psoas cross-sectional area ventral to the β trajectory potentially retracted. (F) An example showing a postoperative axial view of MRI showing the cage’s final location under the β trajectory. Entry point (*). 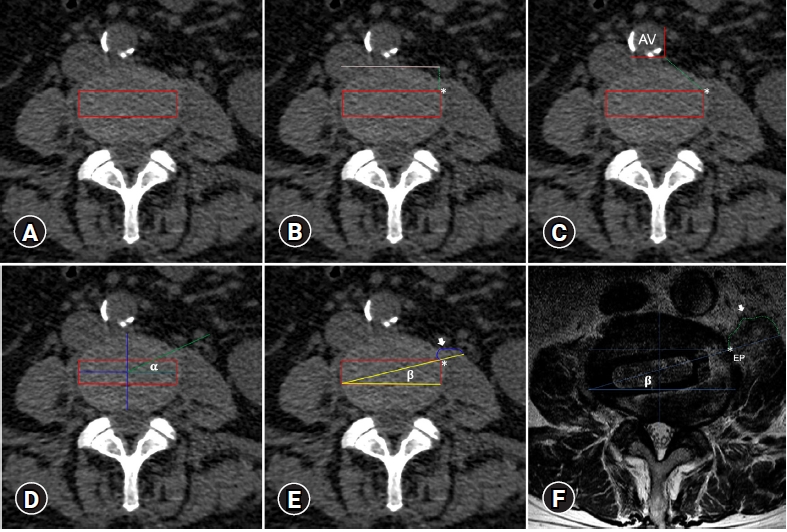
Figure 2.The bar chart demonstrates the longest entry point – anterior vessel distance at the L3-L4 level, followed by L2-L3. The narrowest space was found in L4-L5. The comparison of L4-L5 to the other levels was statistically significant (*). 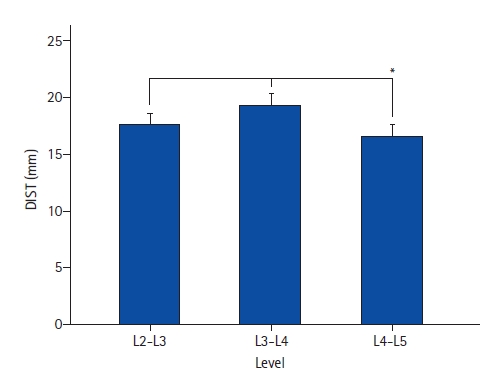
Figure 3.The bar chart shows the total psoas cross-sectional area and the percentage of possible retraction on each level based on the β trajectory. It is evident the biggest area of the psoas at L4-L5 with the most psoas retraction. *Asterisks denote significance. 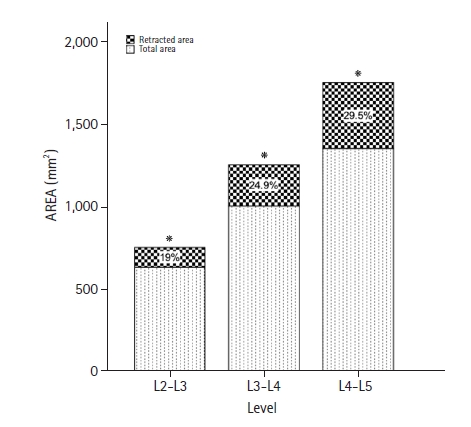
Table 1.Entry point (EP) distances measured
Table 2.Angle measurements of alpha and beta trajectories
REFERENCES1. Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997 22:691–699. discussion 700.
2. Silvestre C, Mac-Thiong JM, Hilmi R, Roussouly P. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J 2012;6:89–97.
3. O’Brien JR. Nerve injury in lateral lumbar interbody fusion. Spine (Phila Pa 1976) 2017;42 Suppl 7:S24.
4. Cummock MD, Vanni S, Levi AD, Yu Y, Wang MY. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine 2011;15:11–18.
5. Li JX, Phan K, Mobbs R. Oblique lumbar interbody fusion: technical aspects, operative outcomes, and complications. World Neurosurg 2017;98:113–123.
6. Quillo-Olvera J, Lin GX, Jo HJ, Kim JS. Complications on minimally invasive oblique lumbar interbody fusion at L2-L5 levels: a review of the literature and surgical strategies. Ann Transl Med 2018;6:101.
7. Davis TT, Hynes RA, Fung DA, Spann SW, MacMillan M, Kwon B, et al. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs in the lateral position: an anatomic study. J Neurosurg Spine 2014;21:785793.
8. Liu L, Liang Y, Zhang H, Wang H, Guo C, Pu X, et al. Imaging anatomical research on the operative windows of oblique lumbar interbody fusion. PLoS One 2016;11:e0163452.
9. Wang Z, Liu L, Xu XH, Cao MD, Lu H, Zhang KB. The OLIF working corridor based on magnetic resonance imaging: a retrospective research. J Orthop Surg Res 2020;15:141.
10. Tao Y, Huang C, Li F, Chen Q. Magnetic resonance imaging study of oblique corridor and trajectory to L1-L5 intervertebral disks in lateral position. World Neurosurg 2020;134:e616–e623.
11. Zhang F, Xu H, Yin B, Tao H, Yang S, Sun C, et al. Does right lateral decubitus position change retroperitoneal oblique corridor? A radiographic evaluation from L1 to L5. Eur Spine J 2017;26:646–650.
12. Julian Li JX, Mobbs RJ, Phan K. Morphometric MRI imaging study of the corridor for the oblique lumbar interbody fusion technique at L1-L5. World Neurosurg 2018;111:e678–e685.
13. Chen X, Chen J, Zhang F. Imaging anatomic research of oblique lumbar interbody fusion in a Chinese population based on magnetic resonance. World Neurosurg 2019;128:e51–e58.
14. Ng JP, Kaliya-Perumal AK, Tandon AA, Oh JY. The oblique corridor at L4-L5: a radiographic-anatomical study into the feasibility for lateral interbody fusion. Spine (Phila Pa 1976) 2020;45:E552–E559.
15. Molinares DM, Davis TT, Fung DA. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs: an MRI study. J Neurosurg Spine 2016;24:248–255.
16. Huang C, Xu Z, Li F, Chen Q. Does the access angle change the risk of approach-related complications in minimally invasive lateral lumbar interbody fusion? An MRI study. J Korean Neurosurg Soc 2018;61:707–715.
17. Boghani Z, Steele WI, Barber SM, Lee JJ, Sokunbi O, Blacklock JB, et al. Variability in the size of the retroperitoneal oblique corridor: a magnetic resonance imaging-based analysis. Surg Neurol Int 2020;11:54.
18. Sato J, Ohtori S, Orita S, Yamauchi K, Eguchi Y, Ochiai N, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J 2017;26:671–678.
19. Lang G, Perrech M, Navarro-Ramirez R, Hussain I, Pennicooke B, Maryam F, et al. Potential and limitations of neural decompression in extreme lateral interbody fusion-a systematic review. World Neurosurg 2017;101:99–113.
20. Mahatthanatrakul A, Kim HS, Lin GX, Kim JS. Decreasing thickness and remodeling of ligamentum flavum after oblique lumbar interbody fusion. Neuroradiology 2020;62:971–978.
21. Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br J Neurosurg 2015;29:705–711.
22. Shiga Y, Orita S, Inage K, Sato J, Fujimoto K, Kanamoto H, et al. Evaluation of the location of intervertebral cages during oblique lateral interbody fusion surgery to achieve sagittal correction. Spine Surg Relat Res 2017;1:197–202.
23. Moro T, Kikuchi S, Konno S, Yaginuma H. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine (Phila Pa 1976) 2003 28:423–428. discussion 427.
24. Le TV, Baaj AA, Dakwar E, Burkett CJ, Murray G, Smith DA, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37:1268–1273.
25. Abe K, Orita S, Mannoji C, Motegi H, Aramomi M, Ishikawa T, et al. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery: perspectives and indications from a retrospective, multicenter survey. Spine (Phila Pa 1976) 2017;42:55–62.
26. Jin J, Ryu KS, Hur JW, Seong JH, Kim JS, Cho HJ. Comparative study of the difference of perioperative complication and radiologic results: MIS-DLIF (minimally invasive direct lateral lumbar interbody fusion) versus MIS-OLIF (minimally invasive oblique lateral lumbar interbody fusion). Clin Spine Surg 2018;31:31–36.
27. Campbell PG, Nunley PD, Cavanaugh D, Kerr E, Utter PA, Frank K, et al. Short-term outcomes of lateral lumbar interbody fusion without decompression for the treatment of symptomatic degenerative spondylolisthesis at L4-5. Neurosurg Focus 2018;44:E6.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||