Accuracy and Clinical Outcomes of Fluoroscopy-Guided and Robotic-Assisted Percutaneous Pedicle Screw Fixation Performed by a Single Surgeon at a Single Center
Article information
Abstract
Objective
Fluoroscopy-guided percutaneous pedicle screw fixation (FGPSF) and its further development, robot-assisted percutaneous pedicle screw fixation (RAPSF), are minimally invasive spinal surgery (MISS) techniques. FGPSF is a standard technique at our hospital, and RAPSF incorporating artificial intelligence has been performed at our hospital since October 2021. This study compared these 2 techniques and analyzed their differences, accuracy, and clinical outcomes based on our experiences.
Methods
This study conducted a detailed analysis of screw accuracy and the clinical outcomes of 2 MISS techniques, FGPSF, and RAPSF. Screw accuracy was evaluated using the Gertzbein and Robbins scale, categorizing placements into grades A–E, with grades A and B considered clinically acceptable. Accuracy was assessed using postoperative computed tomography images for FGPSF and intraoperative O-arm scan images for RAPSF. Clinical outcomes were compared by examining parameters, such as hospitalization duration, C-reactive protein (CRP) normalization period, estimated blood loss (EBL), and preoperative/postoperative visual analogue scale (VAS) scores. Screw-related complications were reviewed. Independent image evaluations by nonparticipating spine specialists ensured objective and reliable assessments.
Results
Both FGPSF and RAPSF demonstrated high rates of clinically acceptable screw placement, with minimal breaches that required no repositioning. The clinically acceptable rates of FGPSF and RAPSF were similar (99.17% and 99.19%, respectively). Both groups also demonstrated similar clinical outcomes. The CRP normalization period, EBL, and ΔVAS (preoperative—postoperative) scores revealed no statistically significant differences between FGPSF and RAPSF. Neither group experienced screw-related complications; however, the RAPSF group exhibited a statistically significant shorter hospital stay than the FGPSF group.
Conclusion
This study compared the accuracy and clinical outcomes of FGPSF and RAPSF. Both methods demonstrated no significant differences in accuracy or clinical outcomes. Spine surgeons selected between the 2 methods based on individual patient needs, and additional research is required to fully understand the practical advantages of each technique in the clinical field.
INTRODUCTION
South Korean society has become an aging society, and concomitantly, the number of patients suffering from degenerative spinal diseases, such as spinal stenosis and spondylolisthesis, has been steadily increasing [1]. Minimally invasive spine surgery (MISS) has been a safe and effective alternative to conventional open spinal surgery [2]. MISS enables spinal procedures with significantly reduced blood loss and soft tissue damage [3]. Fluoroscopy-guided percutaneous pedicle screw fixation (FGPSF) is one of these MISS techniques, whereas robot-assisted percutaneous pedicle screw fixation (RAPSF) is a more advanced form that incorporates artificial intelligence [4]. FGPSF is primarily used as the standard technique for posterior spinal fixation at our hospital. However, we have increasingly adopted the use of RAPSF since the initial application of percutaneous pedicle screw fixation using the CUVIS-spine robotic system (CUREXO, Seoul, Korea) in October 2021 [5]. Consequently, this study aims to compare and contrast these 2 minimally invasive screw insertion techniques, explore their differences, and analyze their respective accuracy and clinical outcomes based on our clinical experiences.
MATERIALS AND METHODS
1. Patient Selection
Two patient groups were investigated, including those who underwent oblique lumbar interbody fusion (OLIF) with FGPSF from April 2017 to December 2020 and those who received OLIF with RAPSF using the CUVIS-spine robotic system from October 2021 to November 2022. We followed the conventional approach that our hospital has been using for OLIF [6]. The screws inserted in both patient groups used Zenius (Medyssey, Jecheon, Korea) with a diameter of 6.0 or 6.5 mm. Both patient groups were evaluated for factors such as bone mineral density (BMD), body mass index (BMI), underlying disease, and medical history (Table 1). Clinical outcomes and radiological assessments were retrospectively analyzed, and relevant data were collected through our hospital’s electronic medical records and the PACS (Picture Archiving and Communication System).
This study was approved by the Institutional Review Board (IRB) of Pusan National University Yangsan Hospital (IRB No. 55-2024-031).
2. Inclusion and Exclusion Criteria
The study included patients with persistent lower back pain and radicular pain that lasted for at least 3 months, as confirmed by preoperative magnetic resonance imaging. Participants were those who did not respond to medical treatment. This study excluded patients with pyogenic spondylitis, traumatic spinal disorder, or neoplasm. This study focused on patients operated on by a single surgeon who had passed a certain point in the learning curve at our hospital. Therefore, both patient groups under investigation underwent surgery by the same surgeon, and cases in which surgery was performed by different surgeons were excluded. Additionally, this study included patients who only underwent one level of OLIF while excluding those who underwent fusion surgery at ≥2 levels, as well as patients undergoing posterior decompressive laminectomy. Stringent inclusion and exclusion criteria were applied to maintain consistent evaluation, particularly in the context of screw fixation methods.
3. Screw Insertion Techniques
1) Procedure for FGPSF
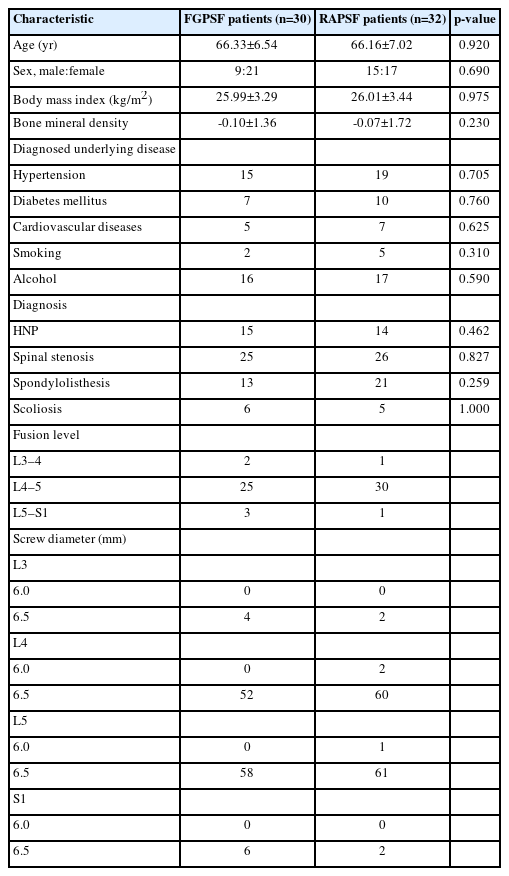
Screw insertion is initiated by verifying a fluoroscopic true anteroposterior (AP) image at the targeted level (Figure 1). A skin incision was made at the lateral boundary of the discernible projected pedicle on the fluoroscopic image. A cannulated needle is placed into the central-lateral boundary of the projected pedicle after blunt dissection through the subcutaneous tissue, fascia, and muscles. Subsequently, the needle was advanced into the pedicle entrance on a fluoroscopic true lateral image (Figure 2). A K-wire is introduced through the cannulated needle to ensure that the cannulated needle does not extend beyond the medial pedicle border on the fluoroscopic true AP image. After removing the needle, the screw is inserted over the K-wire for precise screw fixation (Figure 3).
Verification of a fluoroscopic true anteroposterior image. (A) Confirm the location of the lateral border of the pedicle on the skin. (B) On a fluoroscopic true anteroposterior image, surgeon confirm the lateral border of the pedicle.
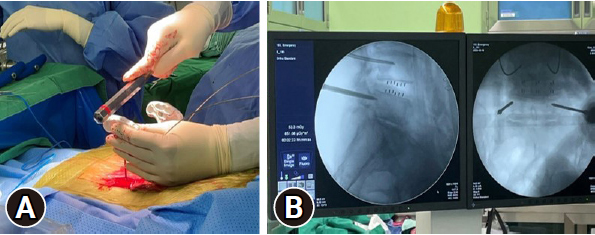
The cannulated needle is advanced into the pedicle entrance on a true lateral fluoroscopic image. (A) The cannulated needle is advanced into the skin. (B) On a true lateral fluoroscopic image, surgeon confirm that the tip of needle is placed at the pedicle entrance.
2) Procedure for RAPSF
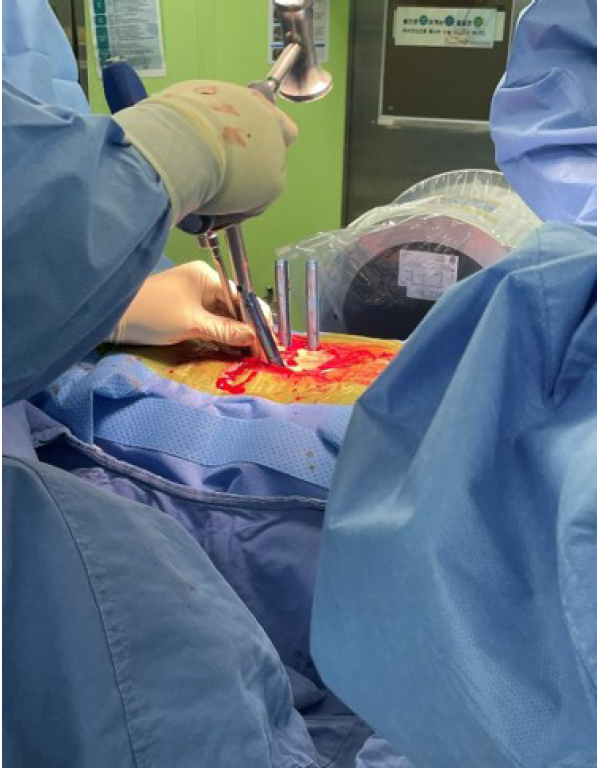
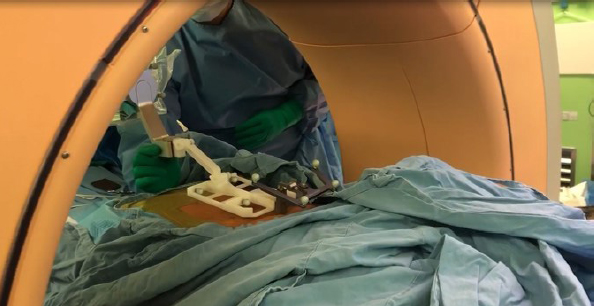
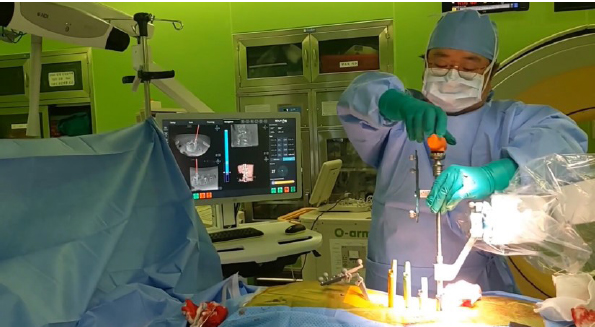
The CUVIS-Spine robotic system, which was domestically designed, incorporates a main console, an optical camera, a floor-mounted robot arm, and a staff console (Figure 4). It uses intraoperative computed tomography (CT) scans for planning, initiated by an O-arm scan on the target vertebrae with a tracker fixed to the 2-level upper spinous process (Figure 5). To install the tracker, we check the level of spinous process 2 levels above the spinous process at the highest level of the screw fixation. And we dissect skin and muscle of the spinous process and then install the tracker. The subsequent steps involve planning and previewing (Figure 6), fixing the robot arm to the planned trajectory, and executing screw insertion into the target vertebrae. The system ensures precision through continuous checks, including skin and fascia release, working corridor creation, drilling, tapping, and trajectory validation with a ball tip probe, thereby improving control and accuracy in the surgical process (Figure 7).
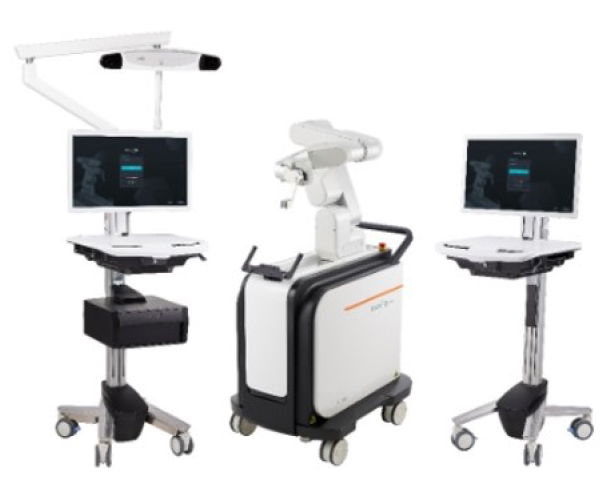
The CUVIS-Spine robotic system (CUREXO, Seoul, Korea). Available from: https://www.curexo.com/english/medical/sub05.php?kind=2.
4. Assessment of the Accuracy of Screw Placement
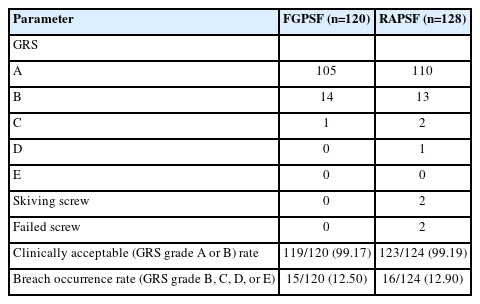
The Gertzbein and Robbins scale (GRS) was used to evaluate the accuracy of the inserted screws in each patient group [7,8]. The grading system for screw placement included grade A for screws placed within the pedicle, grade B for a cortical breach of the pedicle within 2 mm, grade C for a cortical breach of 2–4 mm, grade D for a cortical breach of 4–6 mm, and grade E for a cortical breach of >6 mm. Clinically acceptable grades were considered as A and B [9], and their rates in the 2 groups were investigated. The FGPSF group used postoperative follow-up CT images for screw accuracy evaluation. In contrast, the RAPSF group used intraoperative O-arm scan images conducted in the operating room immediately after screw insertion to assess screw accuracy. Two spine specialists, who were not involved in the surgery, performed image evaluations through duplicate verification.
5. Comparison of the Clinical Outcomes
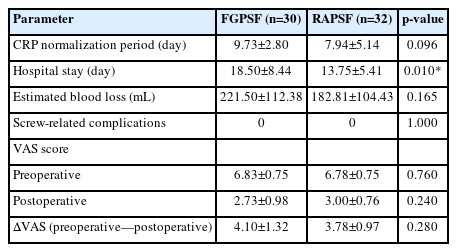
The following parameters were investigated for each group to compare clinical outcomes: length of hospital day from the date of surgery to discharge, duration until postoperative C-reactive protein (CRP) normalization, amount of estimated blood loss (EBL), preoperative and postoperative (at discharge) visual analogue scale (VAS) score, and the occurrence of screw-related complications.
6. Statistical Analysis
IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA) was used for analyses. Normally distributed continuous variables were presented as means with standard deviations, whereas categorical variables were expressed in quantity. Fisher exact test and chi-square test were used for categorical data analysis. Student t-test was utilized for comparing normally distributed independent data. A p-value of 0.05 indicated statistical significance.
RESULTS
This study included 30 and 32 patients in the FGPSF and RAPSF groups, respectively. The average age in the FGPSF group was 66.33±6.54 years, with a male: female ratio of 9:21, BMI of 25.99±3.29, kg/m2, and BMD of -0.10±1.36. The average age in the RAPSF group was 66.16±7.02 years, with a male: female ratio of 15:17, BMI of 26.01±3.44 kg/m2, and BMD of -0.07±1.72. A total of 120 screws were inserted using FGPSF, while 128 screws used RAPSF. RAPSF was successfully used to insert 124 screws, excluding 2 skiving screws and 2 failed screws. Skiving and failed screws, which were deemed risky, were converted to FGPSF and excluded from the analysis of clinically acceptable and breach occurrence rates.
1. Screw Accuracy
In the FGPSF group, 105, 14, and 1 screws were classified as grades A, B, and C, respectively, according to the GRS classification, with no screws graded as D or E. Further, 110, 14, and 1 screws in the RAPSF group were graded as A, B, C, and D, respectively, with no screws graded as E. In both groups, breaches in screws graded C or lower did not indicate a repositioning requirement; therefore, rescue fixation was not performed. The clinically acceptable rates in the FGPSF and RAPSF groups were 99.17% and 99.19%, respectively. Breach occurrence rates were 12.50% and 12.90% in the FGPSF and RAPSF groups, respectively (Table 2).
2. Clinical Outcomes
The clinical outcomes in both the FGPSF and RAPSF groups were as follows: CRP normalization period was 9.73±2.80 days and 7.94±5.14, EBL was 221.50±112.38 mL, and ΔVAS (preoperative—postoperative) were 4.10±1.32 and 3.78±0.97, respectively. No statistically significant differences were observed. Moreover, neither group experienced screw-related complications. Hospital days were 18.50±8.44 and 13.75±5.41 days in the FGPSF and RAPSF groups, respectively, revealing a statistically significant shorter hospital day for the RAPSF group (Table 3).
DISCUSSION
Over the past few decades, spinal surgery has demonstrated remarkable advancements, introducing new technologies to improve surgical outcomes and enhance patient stability [10]. In general practice, FGPSF relied heavily on the surgeon’s anatomical understanding and tactile feedback [11]. A new method called RAPSF has been introduced with the presentation of robotic technology. The adoption of RAPSF in spinal surgery represents a significant technological advancement in surgical accuracy and patient care [12,13]. The technological advancements mentioned are expected to reduce the risk of complications and maintain, on average, the clinical outcomes and accuracy of screws intraoperatively. The actual clinical effects and advantages of RAPSF compared with conventional screw fixation methods remain subjects of ongoing research and debate despite their anticipated benefits [14,15]. Therefore, this study aimed to compare the results of FGPSF and RAPSF in spinal surgery. We compared the clinical outcomes, including screw accuracy, screw-related complications, length of hospital day, EBL, and CRP normalization period. We focused our efforts on obtaining an intuitive understanding of these 2 approaches, and we anticipate that the comparison results will offer insights into the optimal approach for spinal surgery. The accuracy of screw placement is crucial in spinal surgery, which directly affects surgical success and patient safety. The research results reveal no significant difference in accuracy between FGPSF and RAPSF. In a broader context, this study concludes that the accuracy of RAPSF is comparable to the reported accuracy of FGPSF [16,17]. The consistency of the results of this study with the accuracy results of previous analyzes of RAPSF conducted at our hospital indicates excellent reproducibility of the results [5]. This indicates that RAPSF may provide a more consistent accuracy level, thereby further supporting the notion that RAPSF can achieve a level of precision similar to that of FGPSF. Additionally, statistical significance was observed only in the length of hospital days in the RAPSF group, but the average values for CRP normalization period, EBL, and pre- and postoperative VAS were consistently lower in the RAPSF group. This indicates that RAPSF may demonstrate clear advantages over FGPSF in the context of MISS with continued research.
This study reveals that RAPSF represents a technologically advanced method compared with FGPSF, but RAPSF demonstrating clear superiority in terms of accuracy and clinical outcomes over FGPSF cannot be unequivocally stated. Furthermore, the challenges associated with robotic systems, technical complexity, and the cost of robotic equipment may present obstacles to the practical adoption of these technologies in a clinical setting [18,19]. However, continuous additional research is required to determine the practical benefits of these innovative technologies in a clinical environment, as robotic spine surgery systems still possess tremendous untapped potential for further development [20,21]. Effectively using robotic spinal surgery systems in the ever-evolving field of spine surgery requires the integration of such new technologies into clinical practice based on objective research data. This integration will be crucial for the future generation of spine surgeons. Moreover, spine surgeons would be provided with the option to select a surgical approach based on patient preferences and clinical considerations, in the light of the similarities in accuracy and clinical outcomes between the 2 screw fixation methods. In particular, the excellent reproducibility of RAPSF could be an appealing procedural option for young spinal surgeons with limited experience.
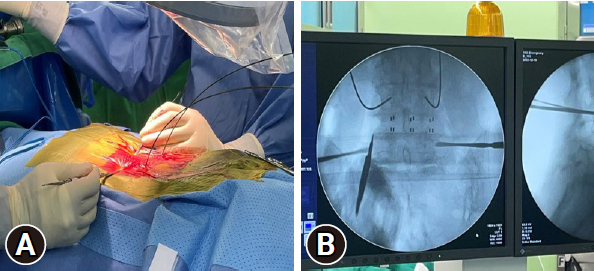
FGPSF posed challenges whereas RAPSF offered advantages based on our experience with specific cases and the previously reported advantages of RAPSF [12,13]. RAPSF demonstrated benefits in cases of poor radiation penetration due to excessive obesity, causing a less visible pedicle. Additionally, instances that involve patients with a stent from abdominal aortic aneurysm repair, where radiation interference from the stent affected precise pedicle visibility, and cases of revision screw fixation in patients who had previously undergone cement-augmented screw fixation, where the existing trajectory was accurately confirmed using the robot (Figure 8), emphasized the advantages of RAPSF.
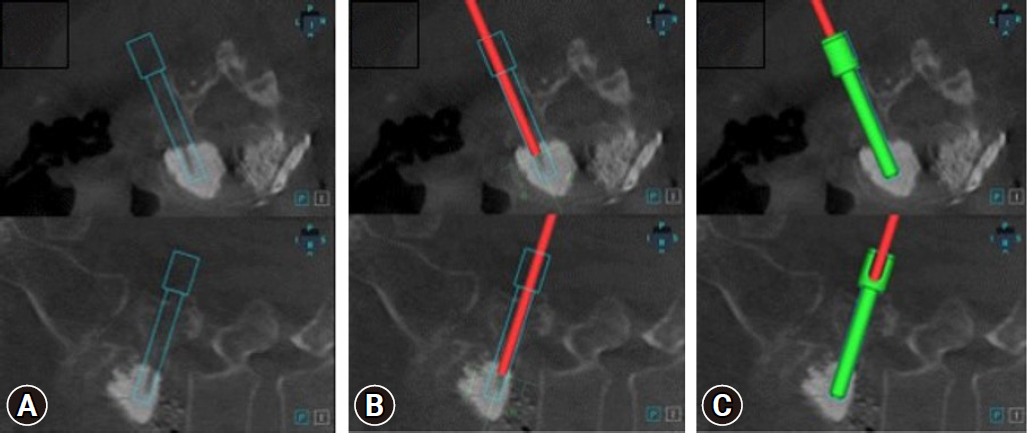
(A) Insertion of screws into the existing cement-filled trajectory planned using a robotic system. (B) Verifying that the existing trajectory matches ball tip probes (red rod) in the robotic system. (C) Accurately inserted screw along the planned path.
This study has several clear limitations. First, the sample size of our study was small. Future studies should involve larger patient populations and randomized controlled trials to more accurately validate these results. Second, only patients who underwent 1-level OLIF were selected as control variables. However, additional research on multilevel fusion cases may be required for a more comprehensive analysis of the differences between FGPSF and RAPSF. Third, the follow-up period for the clinical outcomes of the patient group was short. Long-term follow-ups at 3 months, 6 months, and 1 year and beyond are essential to assess potential differences in clinical outcomes over time. Third this study did not included variables for the time on FGPSF and RAPSF and total radiation exposure. Finally, this study was conducted solely based on the results of research using the CUVIS-Spine robotic system. More convincing results were anticipated to be obtained if a systematic comparative analysis of various robotic systems is conducted.
CONCLUSION
RAPSF has been recently introduced as a contrast to conventional FGPSF, which relies to some extent on the surgeon’s technical proficiency, with MISS advancement. This study compared RAPSF and FGPSF in terms of screw accuracy and clinical outcomes. The results reveal no significant difference in accuracy between RAPSF and FGPSF, with both techniques demonstrating high precision. RAPSF offers technological advancements, but it did not exhibit clear superiority in clinical outcomes compared with FGPSF. This study indicates that spinal surgeons can select between the 2 methods based on patient-specific requirements and clinical considerations. The reproducibility of RAPSF may be particularly advantageous for less experienced spinal surgeons, but further research is warranted to fully understand the benefits of RAPSF in the clinical field.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.