Clinical and Radiologic Outcomes of Biportal Endoscopic Lumbar Interbody Fusion With a Long Polyetheretherketone Cage
Article information
Abstract
Objective
With the latest advances and innovations in field of spine surgery, the new generation of spine surgeons has been increasingly preferring the endoscopic lumbar interbody fusion technique to treat the pathology of lumbar degenerative disease. The aim of this study was to elucidate the clinical and radiologic outcomes of biportal endoscopic lumbar interbody fusion with a long polyetheretherketone (PEEK) cage.
Methods
This study included 40 patients treated by biportal endoscopic lumbar interbody fusion with a long PEEK cage between January 2020 and December 2021. The clinical evaluation was conducted using improvements in visual analogue scale (VAS) and Oswestry Disability Index (ODI) scores. Radiological outcomes were evaluated by changes in disc height and segmental and lumbar lordosis. Fusion was assessed based on computed tomography scans using the Bridewell criteria. Surgical parameters (e.g., operative duration, blood loss and complications) were noted.
Results
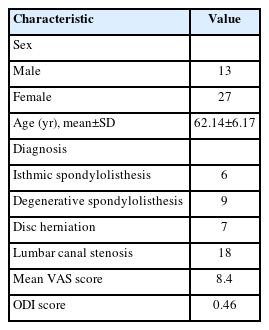
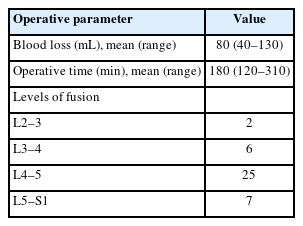
Of the 40 patients in this study, 13 were male and 27 were female. Most patients had significant clinical improvement as indicated by improvements in VAS and ODI scores (p<0.05). Disc height, segmental lordosis, and lumbar lordosis showed significant improvements (p<0.05). The mean surgical duration was 180 minutes, and the mean blood loss was 80 mL. All patients had grade 1 or 2 fusion.
Conclusion
Biportal endoscopic fusion using a long PEEK cage is an excellent option for achieving good interbody fusion when indicated. A long-term follow-up study would be needed to fully clarify the effectiveness of this procedure.
INTRODUCTION
For treating various pathologies associated with lumbar degenerative disease and lessening the low back pain, radiculopathy and disability; a variety of lumbar fusion techniques have been used. Of the many lumbar interbody fusion techniques, transforaminal lumbar interbody fusion (TLIF), which was first described by Harms and Rolinger, has become popular owing to its safety, successful results and better fusion rates. Since TLIF uses a posterior approach and reduces the dural retraction while enabling a direct neural decompression, it is being performed by many spine surgeons with ease. The technique of lumbar interbody fusion has been mentioned to have a higher arthrodesis rate than that of posterolateral onlay fusion technique [1,2]. The traditional open and also tubular retractor system used posterior approach of the lumbar spine adopted for arthrodesis has got a very high soft tissue morbidity which can have a negative impact on the final outcome in patients [3-5]. During the routine surgical exposure of the spine, excessive muscle stripping and retraction causes iatrogenic soft tissue injuries which can be overcome by various techniques of minimally invasive posterior lumbar fusion [6]. Recent studies show that medium to long-term outcomes in terms of clinical advantages for both open and minimally invasive spine surgery is negligible but minimally invasive procedures holds upper hand in perioperative advantages like reduced blood loss, lesser infection rates, lower rate of complications and more importantly lesser time to analgesic independence and can return to work at the earliest [7-14]. Combining the minimally invasive surgery with biportal endoscopic approach for lumbar interbody fusion further decreases the collateral damage from the surgical access and helps with direct view of the pathological site anatomical structures and making it easier for the decompression of nerve roots and removing adhesions with ease [15]. The biggest advantage of endoscope assisted lumbar interbody fusion is that we can have an excellent direct visualization of the vertebral endplate at the time of its preparation and thereby enhances its better standard of preparation, prevention of endplate fractures and helps in achieving a better clinical outcome in the form of fusion and prevention of cage subsidence [12,16,17]. In the current study, authors present their clinical and radiological outcome with unilateral biportal endoscopic lumbar interbody fusion using a long polyetheretherketone (PEEK) cage across the disc space. The operating surgeon believes that spinal fusions successful clinical outcomes depend mainly on: (1) doing a wide decompression, (2) treating instability of any kind with proper instrumentation, and (3) achieving aggressive bony fusion by making use of auto and allograft after proper endplate preparation.
MATERIALS AND METHODS
1. Patient Population
In this prospective study, 40 patients in total (13 men and 27 women; mean age, 62.14±6.17 years) have been enrolled between the time period of January 2020 and December 2021 in Daejeon Woori Hospital. Unilateral biportal endoscopic lumbar interbody fusion with long PEEK cage was done for all patients. The patient information collected was demographics, diagnosis, preoperative visual analogue scale (VAS) and Oswestry Disability Index (ODI) score (Table 1). A thorough documentation of the patient’s clinical history, examination findings, preoperative investigations including imaging studies, operative details, follow-up time, if any postoperative difficulties and functional scores were done. Patients who qualified the inclusion and exclusion criteria set were selected. The patients with nonresolving low back pain with radiculopathy even after giving a minimum of 4 weeks of conservative trial, combination of medical history and magnetic resonance imaging (MRI) rooting for a diagnosis of lumbar degenerative disease especially lumbar spinal canal stenosis (central and lateral recess stenosis) and spondylolisthesis (lower than grade II), involvement of single-segment/level pathology and chronic cases of lumbar degenerative disease not improved even after nonsurgical treatments that failed or were more than 6 months were included while patients with metastatic disease, acute extruded disc herniation, symptoms or signs not correlating to the imaging studies, patients with coagulation abnormalities or those who had previously undergone instrumentation surgery for the lumbar level and those who were not willing to undergo surgery or could not to complete follow-up criteria were excluded.
This study was approved by the Institutional Review Board (IRB) of the Daejeon Woori Hospital.
2. PEEK Cage
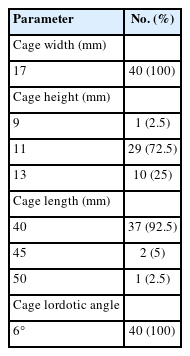
The PEEK cage is a high molecular weight thermoplastic material which has got a modulus of elasticity closer to that of bone thereby resulting in less stress shielding, better transfer of load, decreased chance of subsidence and more importantly has got a higher rate of fusion [18]. Radiologically on computed tomography (CT) scan, the fusion status can be better assessed with a PEEK cage as it is radiolucent. Other added advantage is that it mostly avoids any chance of infection as PEEK is an inert material that resists cell adhesion [19,20]. These cages have metallic markers for identification purpose. The cages used for this study was slightly smaller than the conventional oblique lumbar interbody fusion cages, larger than the posterior lumbar interbody fusion (PLIF) cages and TLIF cages [21,22]. The cage dimensions used in this series were of length (40 mm, 45 mm, or 50 mm), height (9 mm, 11 mm, or 13 mm), width (17 mm), and 6° lordotic angle (Table 2. Figure 1), when compared to the conventionally used PLIF cage (25 mm/12 mm/9 mm/0°) and TLIF cage (10 mm/12 mm/30 mm/0°) sizes [22].
3. Surgical Technique
Patients were given general or epidural anaesthesia and taken in prone position. More often the right-handed surgeon prefers to approach from left side as it makes it relatively easy to get the surgical instruments from the scrub nurse. A right sided approach is however opted in some cases like-in L5–S1 level or those with a high lordotic angle or even when their demands a direct neural decompression of right foraminal stenosis is warranted. Two transverse skin incisions which are approximately 3 cm apart are made, with inferior part of the cranial lamina at midline being the center of upper pedicle and lower pedicle. The cranial incision is for the endoscopic portal while the caudal one is for the working portal. In obese patients, the skin incisions may need to be further lateral. Serial tube dilators are inserted initially so as to make an easy pathway for endoscopic sheath insertion subsequently. These make triangulation at spinolaminar junction. Soft tissue is cleared off by using radiofrequency (RF) probe. Unilateral laminotomy with bilateral decompression is done to achieve central canal decompression. Ipsilateral complete facetectomy done by removing both the inferior and superior articular processes using multiple osteotomies to save the autograft material. The disc space of ipsilateral side is exposed (Figure 2A), epidural vessels coagulation is done precisely. RF probe or Indian knife may be used for performing annulotomy (Figure 2B). After performing annulotomy, pituitary forceps are inserted to remove the disc material (Figure 2C). A meticulous disc space, endplate preparation is done under vision using a Kerrison punch, angled endplate removers, curettes (Figure 2D), and pituitary forceps so as to achieve a good fusion bed. Atmost care is taken for removing most part of cartilaginous endplate without any bony endplate injury so as to prevent any subsidence of cage into the vertebral body. A 30° scope is used for contralateral side endplate preparation, adequate disc material and cartilaginous endplate removal so that the long PEEK cage sits comfortably. Multiple bleeding spots from the bone marks the end of endplate preparation (Figure 2E). Through the working port, trial size of cage is inserted until a proper size is achieved. Using a bone graft funnel and under fluoroscopy guidance, bone grafting is done by compacting it into anterior portion of disc space. Under direct visualization, the long PEEK cage filled with mixture of autograft and allograft is inserted (Figure 2G) after adequate protection of thecal sac and nerve root by a specific cage guider (Figure 2F). By using a cage impactor, the inserted cage is then placed across the prepared disc space (Figure 2H), and confirmed under the fluoroscopy. Percutaneous pedicle screw insertion is done under fluoroscopy guidance after taking the required skin incisions which marks the end of surgery. A 100-mL surgical drain is inserted via working portal skin incision to prevent any sort of complication arising due to postoperative hematoma formation.
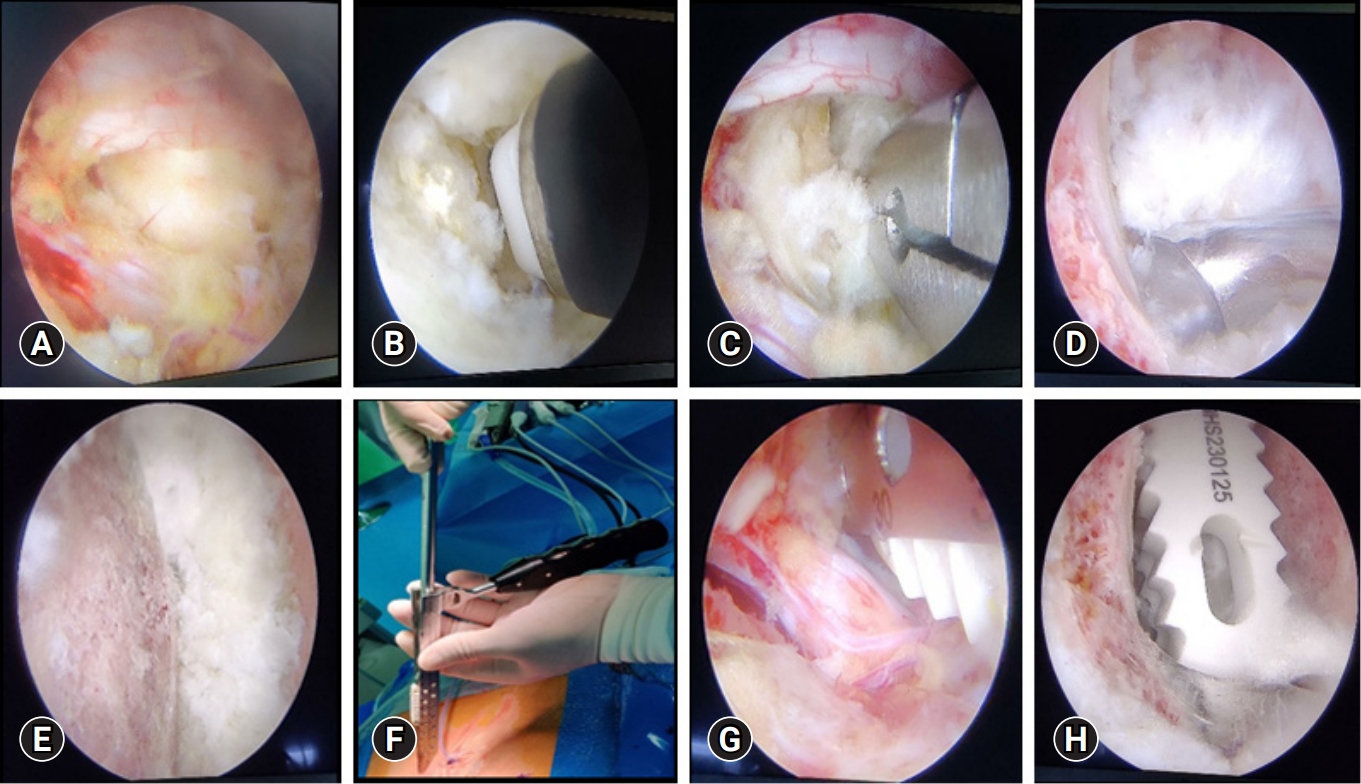
Sequential steps of endplate preparation and cage placement. (A) Exposed disc space. (B) Annulotomy using a specialized radiofrequency probe. (C) Discectomy done with pituitary forceps. (D) Endplate preparation using a curette. (E) Prepared endplate. (F) Specialized instrument (cage guider) for sliding the cage inside and retracting the thecal sac. (G) Insertion of the cage under vision. (H) Long polyetheretherketone cage placed across the disc space.
On day one after surgery, patient is mobilized with physical activity and surgical drain is removed on day 2 postoperatively. Postoperative standing radiographs are taken to see the cage and screw placements. MRI scan done after drain removal shows neural decompression in detail.
4. Clinical Assessment
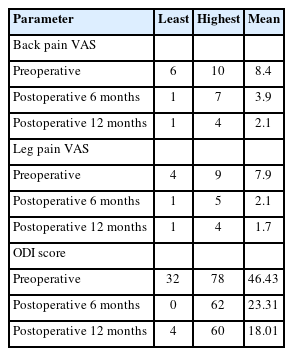
Clinical assessment of all 40 patients included in the study were done on an outpatient basis for back and leg pain VAS scores and ODI scores at post operative 6 months and 12 months. A minimum of 12-month follow-up period after surgery wherein the preoperative VAS scores for overall back and leg pain, and ODI score at the final follow-up showed significant change.
5. Radiological Assessment
The radiological assessment for this study were done using radiographs (x-rays) and CT scan. For determining the disc height, segmental lordosis and lumbar lordosis, x-rays taken at 1 week postoperatively and at 1-year follow-up were included. Segmental height was calculated from 1-week postoperative radiograph was compared to the final follow-up radiograph to assess for any cage subsidence. A difference of >2 mm if present between the two was to be considered positive for subsidence [23]. Fusion outcome was assessed by using CT scan and the scan parameters were similar for all the patients. This was preferred as CT scans have better reliability in assessing bony fusions [24,25]. At a follow-up period of minimum of 12 months, the bony fusion was radiologically assessed using Bridewell fusion grading system. The rate of fusion calculated was sum total of grades 1 and 2.
Criteria for fusion status assessment was discussed by investigators and also by an independent musculoskeletal radiologist. The assessment of fusion was initially done independently by each investigating individual and the radiologist. Those cases which were doubtful were reviewed conjointly to reach a consensus. Grading score on CT scan were done according to the guidelines mentioned in Table 3.
6. Statistical Analysis
For categorical variables, Wilcoxon sign-rank test was used to find the mean and standard deviation. A p-value of <0.05 was considered statistically significant. The statistical analysis was performed using the R ver. 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
In our study, 40 patients in total were included which comprised of 13 males and 27 females. Majority of patients had significant clinical improvement as indicated by improvement in VAS and ODI scores (Figure 3).
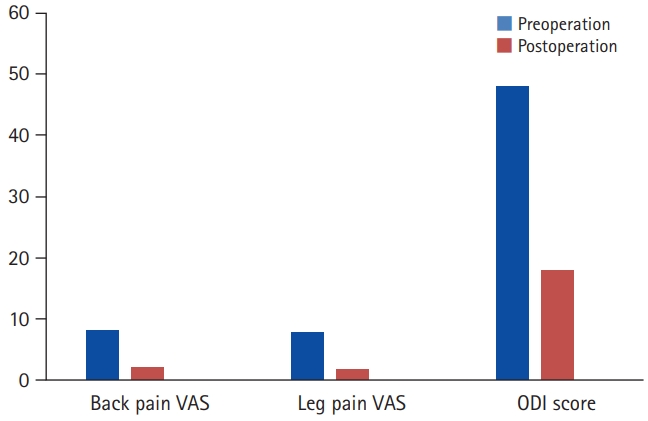
Comparison of preoperative and final clinical outcomes. VAS, visual analogue scale; ODI, Oswestry Disability Index.
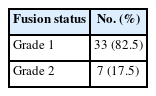
Out of the total 40 patients, 33 patients (82.5%) had grade 1 fusion while 7 patients (17.5%) had grade 2 fusion in our study (Table 4). The mean follow-up was 14.3 months. Mean surgical duration was 180 minutes (range, 120–310 minutes) while mean blood loss compounded to 80 mL (range, 40– 130 mL) (Table 5). There was an average hospital stay of 5 days (range, 3–12 days). For all cases, bilateral pedicle screw fixation was done and there did not arise any need for converting this endoscopic procedure to an open surgery.
On postoperative follow-ups, all patients had significant symptom relief. The patients were gradually able to increase their activity levels and could resume their full activities by 3 months after surgery. The VAS and the ODI were used for quantifying the outcomes. There was a decrease in the average back pain and leg pain VAS score from 8.4 and 7.9 preoperative to 2.1 and 1.7 postoperative respectively at the end of 12-month follow-up. A decrease was also noted in the ODI score from 46% preoperatively to 18% at 12-month follow-up (Table 6).
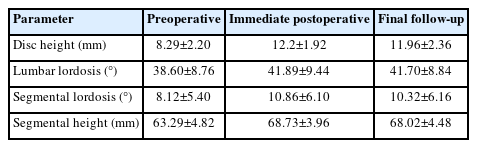
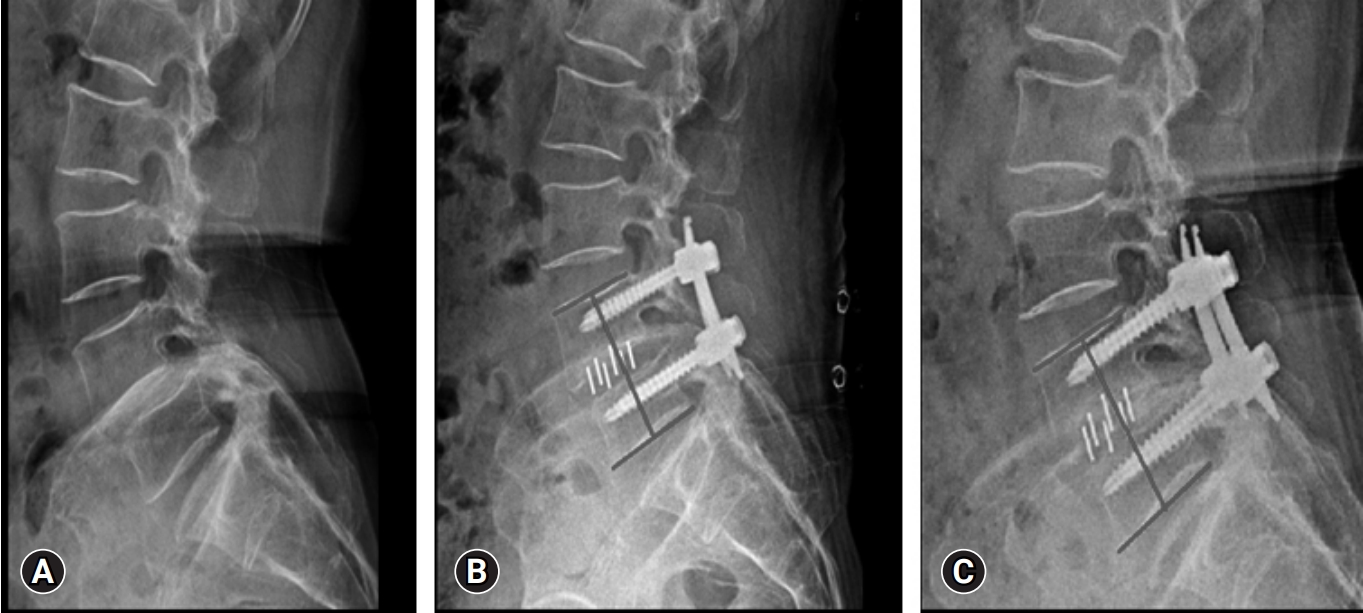
Between 2–4 weeks on an average postoperatively, the narcotic use was discontinued. Majority of the cases CT scan at 12 months minimum follow-up, appeared to have achieved solid radiographic fusions (Figure 4-6). This was determined by trabecular bony bridging presence, less than 3° motions on flexion–extension views, and intact hardware. There was significant improvement in the postoperative radiographic parameters like disc height (from preoperative 8.29±2.20 to final follow-up 11.96±2.36, p<0.05), segmental height (from preoperative 63.29±4.82 to final follow-up 68.10±4.48, p<0.05), segmental lordosis (from preoperative 8.12±5.40 to final follow-up 10.32±6.16, p<0.05) and also lumbar lordosis (from preoperative 38.60±8.76 to final follow-up 41.70±8.84, p<0.05) (Table 7). Subsidence of cage was noted in one patient in the study (Figure 7).
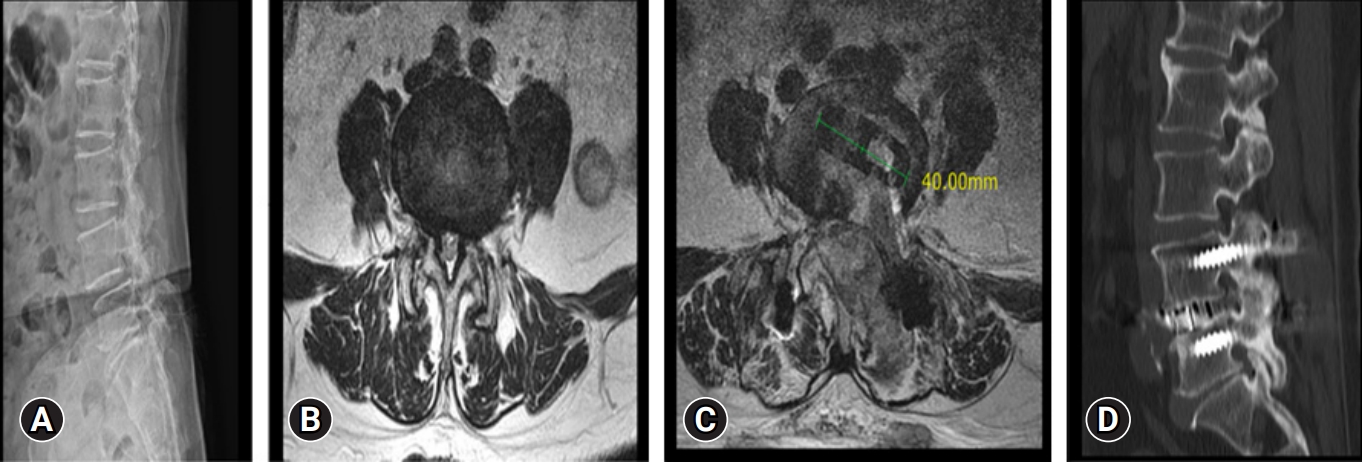
Preoperative and postoperative imaging of a 64-year-old female patient. (A) A lumbar spine x-ray shows degenerative spondylolisthesis at L4–5. (B) Magnetic resonance imaging (MRI) axial cut showing canal compromise. (C) Postoperative MRI axial cut showing decompressed spinal canal with cage in situ at disc space. (D) Postoperative computed tomography showing good bony fusion at the operated L4–5 level.
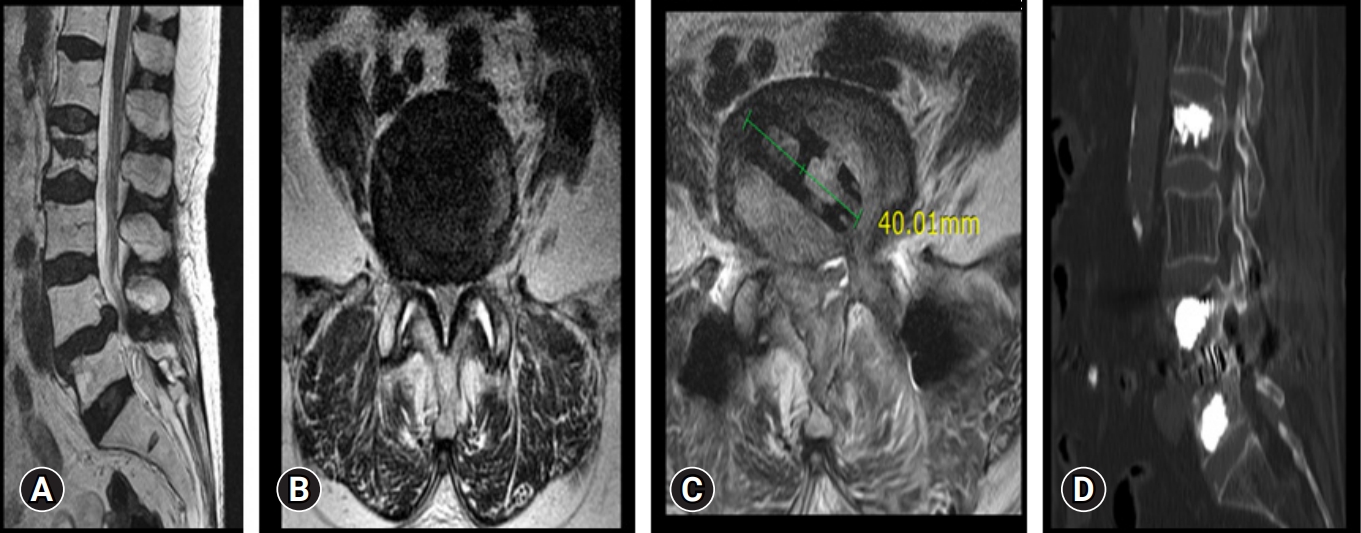
Preoperative and postoperative images of a 72-year-old female patient. (A, B) Magnetic resonance imaging (MRI) sagittal and axial cuts, showing L4–5 disc degeneration and spinal canal stenosis. (C) Postoperative MRI axial cut showing a decompressed spinal canal with the cage in situ. (D) Postoperative computed tomography showing good bony fusion at the operated L4–5 level.
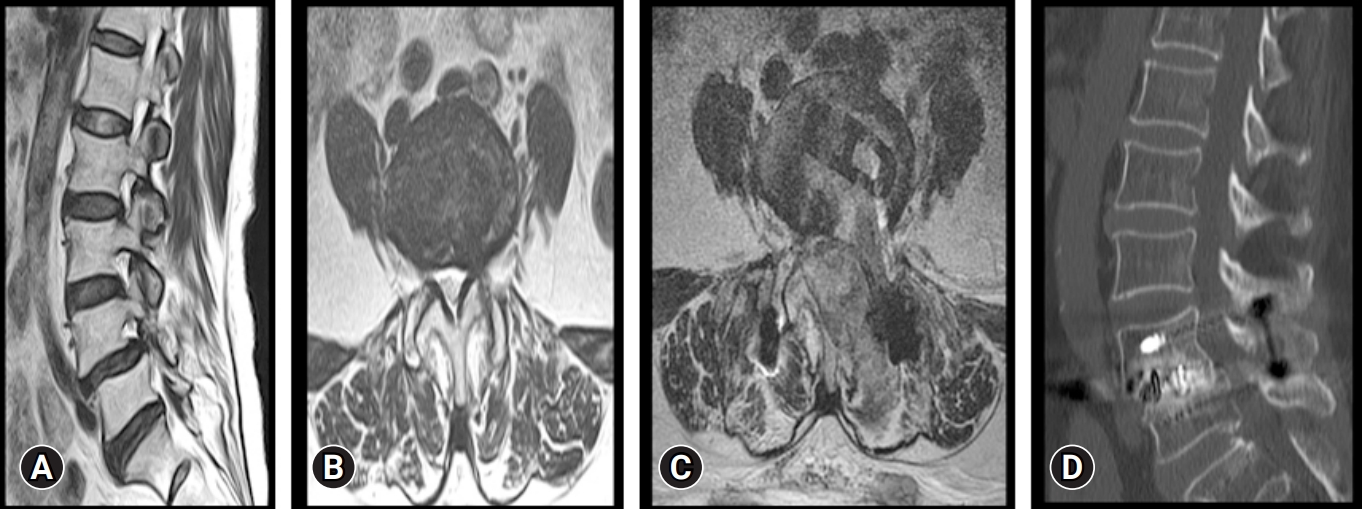
Preoperative and postoperative imaging of a 56-year-old patient. (A) Magnetic resonance imaging (MRI) sagittal view of the lumbar spine, showing lysis at L4 with grade 1 spondylolisthesis of L4 over L5. (B) MRI axial cut showing foraminal stenosis. (C) Postoperative MRI axial cut showing transverse cage placement along the disc space. (D) Postoperative computed tomography scan showing good graft incorporation at the operated L4–5 level.
DISCUSSION
The traditional methods of posterior decompression and fusion continue to be the cornerstone of surgical options for treatment of lumbar degenerative disorder. But, these open surgical dissections denervated all core group of paraspinal muscles which remains the main reason for postoperative back pain and muscle atrophy at the operated segment [26]. In contrast to the conventional open surgeries of spine, endoscopic surgery inflicts minimum muscle damage [27]. Most importantly, decreasing the multifidus muscle damage is an important factor in keeping the spinal segment stability [28]. In this study series, none of the patients required any need for any blood transfusions intraoperatively, demonstrating the procedure is associated with low blood loss and also the patients had significantly less postoperative pain, thereby indicating the minimal invasiveness and advantage of endoscopic lumbar interbody fusion technique.
Chances of endplate damage at the time of fusion bed preparation is a major concern in interbody fusion surgeries [29-31]. But with the usage of endoscope providing a direct visualization of the endplates at the time of preparation has been a major advantage in preparing an excellent fusion bed and preventing accidental endplate damage which can later result in many postoperative complications. With this highly magnified endoscopic vision, it has become relatively easy in separating and removing the cartilaginous endplate from osseous endplate.
There are lot of literatures available now which embarks upon on the merits of endoscopic spine surgery in the treatment of various degenerative spine conditions but the literatures discussing about endoscopic method to fusion is considerably very less. The interbody fusion using a uniportal endoscope via trans-Kambin and facet sparing approach had given appreciable results but a lot of study has reported the incidence of injury to the exiting nerve root and also cage subsidence on patient follow-up in the postoperative period which mostly attributes to the relative difficulty in mastering the new challenging technique of uniportal endoscopic surgeries as it is all together a new dimension whereas these difficulties can be easily overcome in short span of time for surgeons with biportal endoscopic technique as it is more or less similar to the microscopic or minimally invasive TLIF (MIS-TLIF) surgeries which majority of the spine surgeons are familiar with. As a result of this there may be more chances of suboptimal fusion bed preparation or endplate damage occurring during the early learning phase of practice. The interbody fusion using a biportal endoscopic approach after doing a facetectomy has got good acceptance among the spine surgeons as it gives a favourable clinical outcome when compared to microscopic tube assisted fusion surgeries [32].
One of the major advantages of Biportal TLIF surgery is this very thing being the basic principles similar to the MIS-TLIF surgery, thereby inserting a large sized cage with ipsilateral traversing root retraction is very much possible which may be difficult for an inexperienced surgeon performing a uniportal endoscopic lumbar interbody fusion as uniportal endoscopic surgery has a steep learning curve [33].
With many new advents in minimally invasive spinal surgery, there has been considerable decrease in the tissue/muscle damage, blood loss, rehabilitation time, and hospital stay in comparison to conventional open surgery. Different kinds of minimally invasive techniques with good clinical results have been developed owing to the recent advances in optics, endoscopic strategies for addressing lumbar degenerative pathologies especially for discectomy and decompression [29,34-37]. Various techniques for posterolateral, lateral, posterior interbody fusion and MIS-TLIF using a tubular retractor had been gaining popularity as minimally invasive procedures during all these years. Biportal endoscopic procedure will surely run ahead of these in coming years due to its similarity in the surgical steps and instruments to that of open surgery.
All the endoscopic spine surgery techniques have got its own learning curve although it looks very simple [9,38,39]. Due to 2 different working and scopic portals in biportal endoscopic approach to spine, triangulation is the very basic skill needed like that in knee or shoulder arthroscopy. The biportal approach offers a very clear and magnified vision, better identification of microanatomy, good bleeding control, decreases any chance of infection and also less radiation exposure.
The biportal endoscopic approach can also easily address any sort of complications that are bound to occur intraoperatively. The dural tear can be managed by using a tachosil patch or even by suturing it. By achieving a good hemostasis and establishing a drain insitu negates the chances of postoperative hematoma formation, the adequacy of nerve decompression can be checked by a nerve hook or probe for the freeness. The main limiting factor associated with our study is the relatively short duration follow-up, so the possibility of studying various late onset complications including the occurrence of adjacent segment disease could not be included. There are few literatures which remarks suggests that abnormality in the sagittal balance parameters is an important factor in developing an adjacent segment degeneration following a lumbar fusion [40]. Hence a long-term follow-up including these various factors also is needed to authorize the complete effectiveness of the procedure.
CONCLUSION
Unilateral biportal endoscopic limbar interbody fusion using long PEEK cage is an excellent option for achieving interbody fusion when indicated. Still a long term, large scale, and multicenter prospective randomized control trial are necessary to authorize the complete effectiveness.
Notes
Conflicts of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.