Proliferative Myositis of the Erector Spinae Mistaken for Malignancy
Article information
Abstract
We report the case of a 56-year-old woman who presented with a enlarging, painful upper back mass that had developed 4 days ago. The patient was referred to the Emergency Department from a primary care facility after ultrasonography showed an ill-defined mass-like lesion in the back muscle with potential for malignancy. Magnetic resonance imaging and ultrasonography at Eunpyeong St.Mary’s Hospital revealed an ill-defined hypervascular lesion longitudinally oriented in the left erector spinae. The initial differential diagnosis included an inflammatory mass such as proliferative myositis, sarcoidosis, low-grade lymphoma and low-grade infection. The patient underwent ultrasound-guided biopsy with pathology confirming proliferative myositis. Proliferative myositis is a rare benign proliferation of the skeletal muscles that can be mistaken for a malignancy due to its rapid progression.
INTRODUCTION
Proliferative myositis is indeed a rare, benign tumor that can resemble malignant conditions such as sarcoma due to its sudden onset and rapid growth [1]. While the exact incidence of proliferative myositis is unknown due to its rarity, it is generally observed more frequently in middle-aged adults but can occur at any age [1]. Typically, patients with proliferative myositis present with a solitary mass in the proximal limbs, shoulders, or arms, although rare cases involving the head and neck region have been reported [2,3]. We present a rare case of proliferative myositis in the erector spinae muscle which was initially mistaken as malignancy. To the best of our knowledge, there is no existing literature reporting proliferative myositis specifically developed in the erector spinae muscle.
CASE REPORT
A 56-year-old female without comorbidity presented with sudden onset of mid-back mass, just to left of the midline at 4 cm below the tip of the scapular bone. The mass grew rapidly over the period of 4 days accompanied by local tenderness and heat without discoloration. There was no history of trauma or iatrogenic injection. She denied having systemic inflammation signs such as fevers, chills or night sweats. A local physician referred her to our Emergency Department after ultrasonography showing an ill-defined mass-like lesion in the back muscle with potential malignancy (Figure 1).
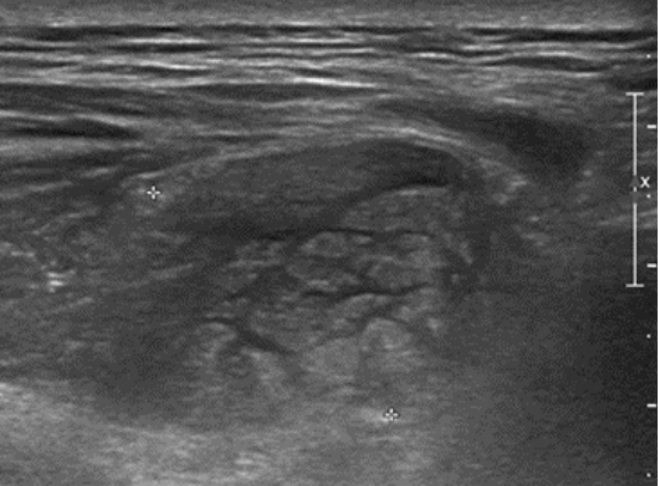
Local ultrasonography showing an ill-defined hyperechoic mass-like lesion with internal hypoechoic streaks in the back muscle.
Laboratory finding including complete blood count, blood chemistry, and serum inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein were within normal range. The thoracic magnetic resonance imaging (MRI) scan demonstrated a 2.5 × 2.5 × 6-cm-sized an ill-defined longitudinal swelling of the inner portion of left erector spinae muscle with low signal intensity (SI) on T1 and high SI on T2-wighted image compared with adjacent skeletal muscle. After gadolinium enhancement, the lesion showed diffuse enhancement with preservation of muscle fascicular structure. Thickening and enhancement of overlying fascia along the left flank were also noted (Figure 2). An initial differential diagnosis was an inflammatory mass of erector spinae muscle including proliferative myositis, sarcoidosis, low-grade lymphoma, fibromastosis, and other types of low-grade infection.
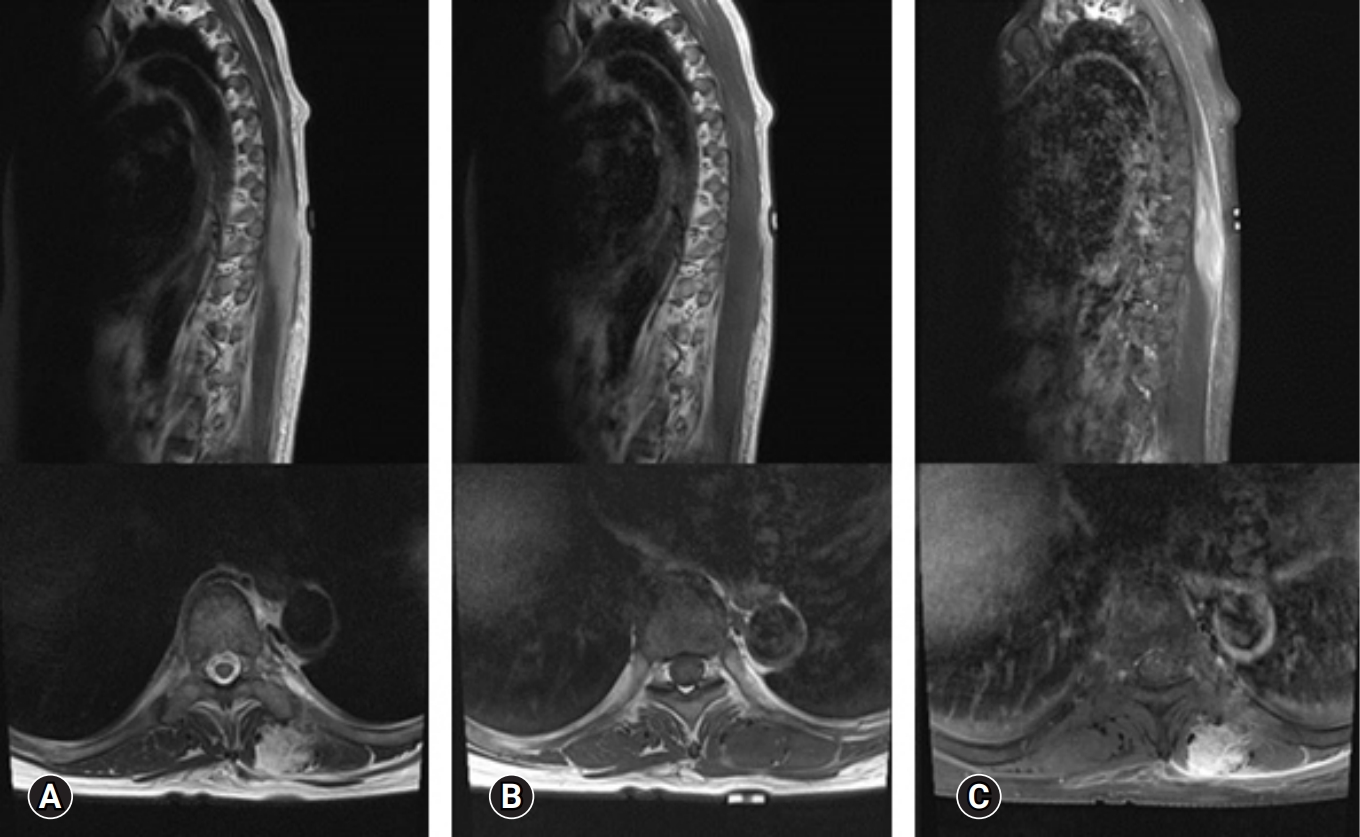
Thoracic magnetic resonance imaging demonstrated a 2.5 × 2.5 × 6-cm ill-defined longitudinal swelling of the inner portion of the left erector spinae muscle with iso-signal intensity (SI) on T1-weighted images (A) and high SI on T2-weighted images (B) compared with the adjacent muscle. After gadolinium enhancement (C), diffuse enhancement of the mass and investing fascia, with preservation of the fascicular structure of the muscle, was identified.
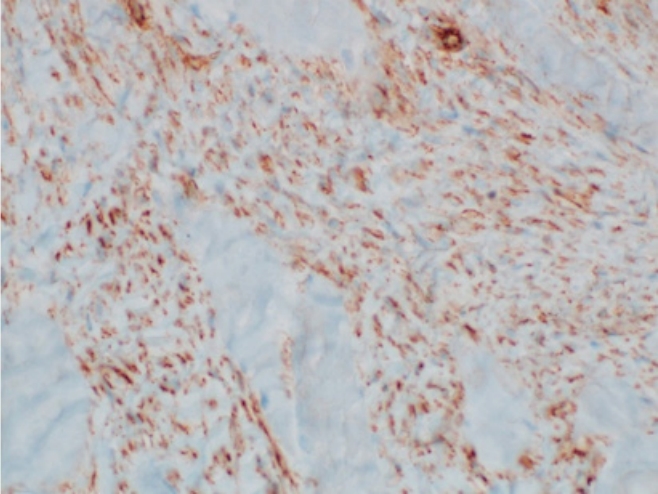
On the ultrasound, the palpable mass was ill-defined hyperechoic lesion with internal hypoechoic streaks in the left paraspinal muscle with mild edema of investing fascia and mild increased vascularity. Ultrasound-guided biopsy was performed by a radiologist with 18 years of experience in musculoskeletal radiology using 18-gauge Stericut needle (Figure 3). Microscopic examination revealed fibroblasts and myofibroblasts surrounding individual skeletal muscle fibers creating the characteristic checkerboard pattern under hematoxylin and eosin stain at low magnification (×40, ×100; Figure 4). At high magnification, plump fibroblasts and myofibroblasts with collagenous stroma and occasional large ganglion-like cells with abundant amphophilic cytoplasm were observed (×400, Figure 5). Immunohistochemically, the atypical spindle cells were positive for actin, but negative for desmin and S-100 protein (Figure 6). The atypical cells also displayed cytoplasmic staining of beta-catenin, which can exclude possible desmoid fibromatosis showing nuclear beta-catenin staining (Figure 7). Based on these microscopic and immunohistochemical findings, a diagnosis of proliferative myositis was made.
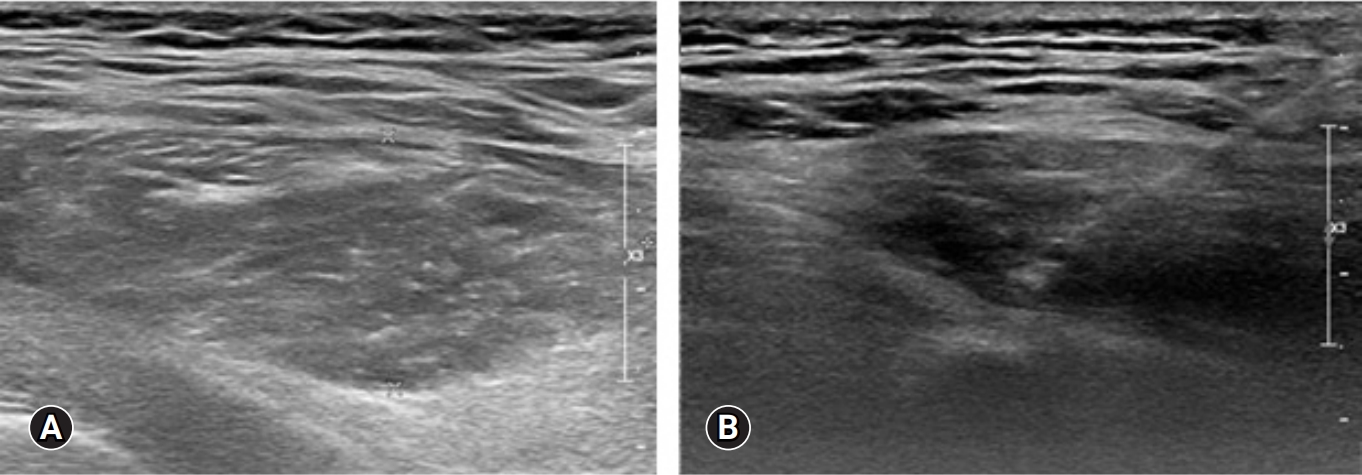
Sono-guided biopsy was performed in the fusiform ill-defined hyperechogenecity of left paraspinal muscle (A) by the experienced radiologist using 18-gauge Stericut needle (B).
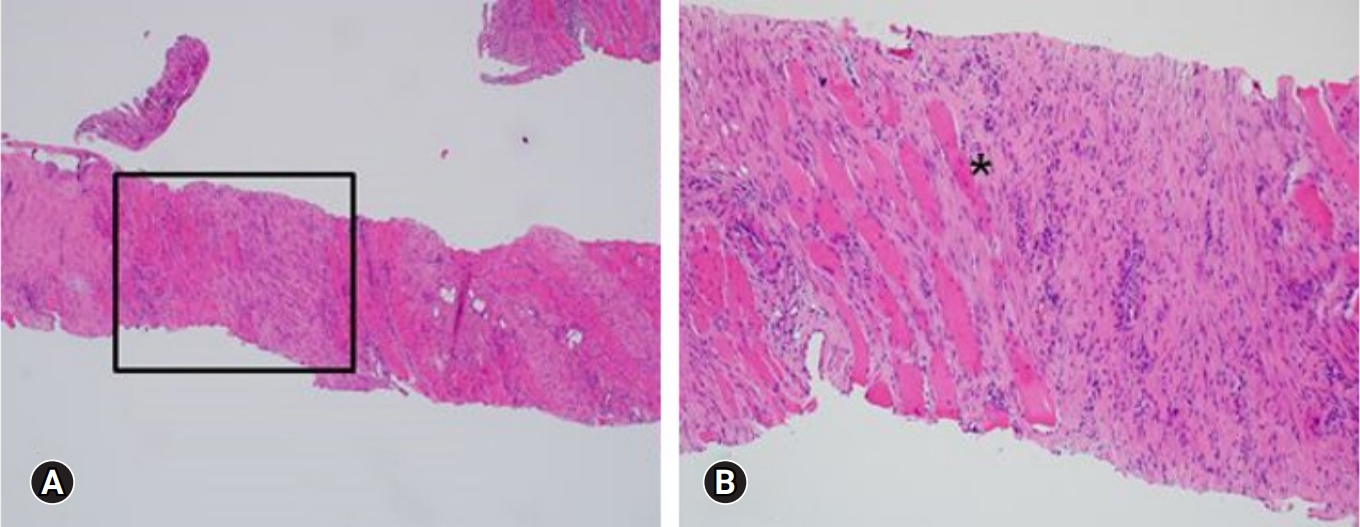
A histologic examination of the lesion revealed fibroblasts and myofibroblasts surrounding individual skeletal muscle fibers, creating the characteristic checkerboard pattern (asterisk; hematoxylin and eosin stain, ×40 [A], ×100[B])
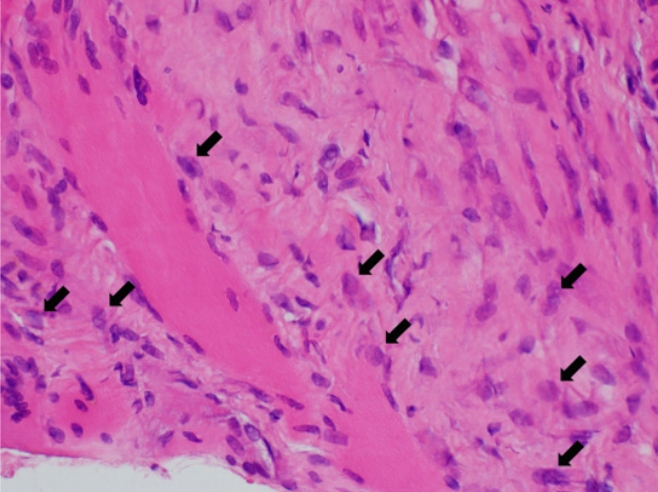
At high magnification, the atypical spindle cells were plump fibroblasts and myofibroblasts with collagenous stroma. There were also occasional large ganglion-like cells (black arrows) with abundant amphophilic cytoplasm (hematoxylin and eosin stain, ×400).
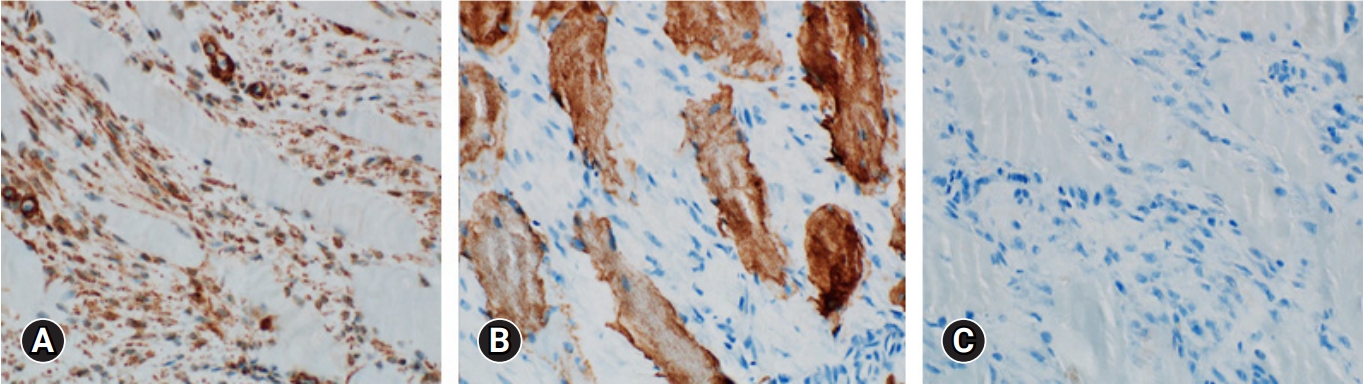
Immunohistochemical analysis of the atypical spindle cells showed positivity for actin (x100, A), but negativity for desmin (x100, B) and S-100 protein (x100, C).
Close observation with oral nonsteroidal anti-inflammatory drugs medication was performed at outpatient clinic. Complete resolution of palpable mass was observed after 2 weeks. At 4-month follow-up, the patient was doing well with no evidence of recurrence. MRI with gadolinium enhancement demonstrated markedly decreased inflammatory lesion with minimal residual linear intramuscular edema at the left erector spinae muscle (Figure 8).
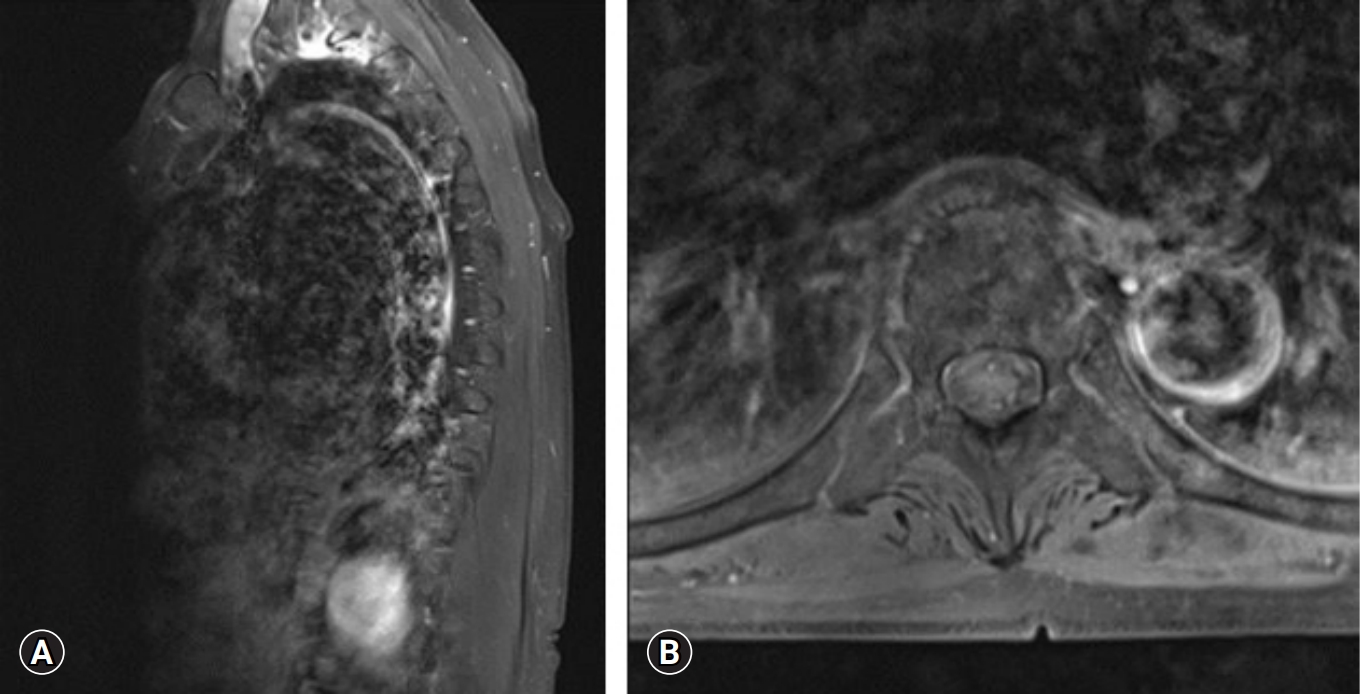
Follow-up magnetic resonance imaging with gadolinium enhancement demonstrated a markedly decreased inflammatory lesion with minimal residual linear intramuscular edema in the left erector spinae muscle. (A) Sagittal cut image. (B) Axial cut image.
Ethics statement: Written informed consent for publication was obtained from the patient.
DISCUSSION
Proliferative myositis is a rare, benign tumor that can mimic malignant conditions such as sarcoma with its sudden onset and rapid growth [1]. According to the literatures, exact incidence of proliferative myositis is unknown as it is a highly rare condition, however, it is generally agreed that it can occur at any age but is more frequently observed in middle-aged adults. Patients typically present with a solitary mass located in the proximal limbs, shoulders, or arms, however, in rare cases, head and neck region [2,3]. In the best of our knowledge, this is the first report of proliferative myositis developed on erector spinae muscle in literature. Although several theories have been suggested, the etiology is yet unknown [1]. Some of the inciting factors proposed are muscle trauma, local ischemia, paracrine myopathy, and genetic anomaly [4,5]. The signs and symptoms depend upon the tumor’s characteristics and usually present as a painless fast-growing lesion that appears centered within skeletal muscle without signs of overt inflammation [5,6].
Radiographic examination is warranted for the diagnosis and known differential diagnoses include soft tissue malignancies such as rhabdomyosarcoma, myositis ossificans and nodular fasciitis [1,5]. Ultrasonography is particularly valuable as an initial imaging modality. Pathognomonic findings are “scaffolding” or “steel cable-like” pattern demonstrating hypoechoic geometric lines within a dense, hyperechoic mass [2,7]. Computed tomography imaging shows a nonspecific poorly demarcated intramuscular lesion with irregular borders [7]. Typical MRI findings are a hypointense or isointense signal on T1-weighted sequences with homogeneous enhancement and strongly hyperintense signal on T2-weighted sequences compared to surrounding muscles [1,8]. As laboratory and imaging examinations are nonspecific, pathological examination by fine needle aspiration biopsy or incisional biopsy is required for a definitive diagnosis histologic characteristic findings include a “checkerboard” pattern of myofibroblasts infiltrating surrounding muscle fibers, ganglion-like basophilic giant cells, and absence of atypical mitosis [5,6,9].
When considering the differential diagnosis for myositis versus malignancy, there are a few key differences to consider. Myositis, an inflammatory condition of the muscle, may present on MRI as increased SI on T2-weighted and short tau inversion recovery images, indicating inflammation or edema. This would typically involve the muscle fibers themselves which often affects muscles symmetrically and unlike a tumor, myositis does not typically create a mass effect. On the other hand, malignancy often present as a discrete mass and this might be seen as a well-defined or irregular mass that's distorting the normal architecture of the surrounding tissue on the MRI. They also often have a heterogeneous signal on MRI due to areas of necrosis, hemorrhage, or different cell densities within the tumor and after administration of contrast, malignant lesions often show enhancement due to the increased vascularity.
Proliferative myositis and myositis ossificans are both benign conditions of the muscles, but their presentation and characteristics can be quite different. Myositis ossificans is a benign, self-limiting process characterized by heterotopic ossification of the muscles. Typically is often associated with a history of trauma, although it can occur without any apparent cause. On radiographic images, myositis ossificans appears as a well-demarcated mass with central calcification. The calcification is often peripheral initially and then becomes more central as the condition progresses. On MRI, myositis ossificans shows heterogeneous SI with a surrounding rim of decreased SI, which represents the mature bone. After contrast administration, there's often peripheral enhancement.
Treatment options for proliferative myositis include watchful waiting or surgical excision. Watchful waiting is usually favored due to its benign nature and the potential for spontaneous resolution [8,9]. Although the mass grows rapidly, complete resolution occurs over weeks to months. If the tumor continues to progress, affects the patient's daily life, alters cosmetic appearance, or shows high suspicion for malignancy despite work up, then surgical resection is recommended [5,6,10]. Radical excision should be avoided as there have been no reports of either recurrence or malignant transformation in literatures [5,10].
CONCLUSION
Proliferative myositis is a rare, self-limiting, benign tumor of the skeletal muscle which can easily be mistaken for malignancy due to its rapid presentations. Its diagnosis can be difficult and, in many cases, diagnosis is not confirmed until after surgical resection. Fine needle biopsy is helpful in the diagnosis of proliferative myositis, thus avoiding unnecessary surgical trauma and costs. We present a rare case of proliferative myositis of the erector spinae muscle, not previously described in the literature.
Notes
Conflicts of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.