Prevalence of Cervical and Thoracic Spinal Disease: A Systematic Review
Article information
Abstract
Objective
This study aimed to comprehensively assess the prevalence and distribution of degenerative cervical and thoracic diseases with compression of the spinal cord, such as disc herniation (TDH) or hypertrophied ligamentum flavum causing stenosis, by reviewing the literature.
Methods
We searched PubMed/MEDLINE to identify articles on the prevalence of degenerative diseases with compression of the spinal cord in the cervical and thoracic spine. The levels of evidence were classified according to the NASS 2005 method. We selected articles containing information on the prevalence of degenerative cervical and thoracic diseases.
Results
We identified 358 articles. Thirty-eight met our criteria, with evidence ranging from levels I to V. The prevalence of asymptomatic spinal cord compression lesions was found to be relatively high in elderly people with underlying conditions. Non-traumatic spinal cord injuries are caused by various degenerative diseases involving spinal cord compression, such as cervical myelopathy, ossification of the posterior longitudinal ligament, and ossification of the ligamentum flavum, and are observed in more than 50% of patients with lesions in Japan and the United States, more than 30% in Europe, and more than 20% in Australia. Regarding thoracic lesions, a prevalence of 5% to 10% has been reported for various spinal cord compression lesions such as herniated disc, ossification of the posterior longitudinal ligament, and ossification of the ligamentum flavum.
Conclusion
Spinal cord compressive lesions appear not to be rare in the cervical and thoracic spine. The radiographic findings of various stenotic lesions must be well understood and correlated with clinical symptoms before treatment decisions.
INTRODUCTION
An increase in the elderly population worldwide has led to an increase in spinal degenerative diseases and spinal surgery. Most of the surgeries are performed in the lumbar region, but recently, degenerative changes in the cervical and thoracic spine are increasing [1]. Additionally, to avoid unexpected neurological deterioration due to the coexistence of upper spinal compression lesions, it is essential for surgeons to be aware of the prevalence and distribution of lesions in lumbar degenerative diseases.
In addition to the lumbar region, stenosis can occur in the cervical and thoracic regions, and can occur simultaneously. However, the literature on predictive radiologic findings of cervical stenosis related to thoracic stenosis is very scarce. The radiographic features of symptomatic cervical and thoracic stenosis are clearly more important than those of asymptomatic stenosis.
However, unlike symptomatic cervical and thoracic stenosis, there is no clinical reason to undergo radiographic examination for asymptomatic stenosis. However, severe radiographic stenosis can be present without symptoms [2,3]. In most cases, these “silent” stenosis do not cause clinical sequelae.
However, there have been cases of paralysis of spinal origin after anesthesia or sleep in patients with asymptomatic spinal stenosis [4,5].
Ossification of the posterior longitudinal ligament (OPLL) and yellow ligament (OLF), which occur more frequently in the cervical and thoracic spine than in the lumbar spine, are characterized by replacement of the posterior longitudinal ligament and yellow ligament by ectopic new bone formation, respectively [6,7].
OPLL often causes a narrow spinal canal and has been recognized as one of the causes of cervical myelopathy and/or radiculopathy [6,8]. OLF is also well known as one of the causes of thoracic myelopathy by compressing the spinal cord from the posterolateral side [7].
In addition, disc herniation in the cervical and thoracic region accompanies various neurologic symptoms of radiculopathy and myelopathy depending on the location and degree of herniation.
This study aims to review the literature on the prevalence of symptomatic degenerative diseases that cause neurological symptoms in the cervical and thoracic spine, and to report the analysis as a systematic review and narrative analysis.
MATERIALS AND METHODS
We used the Preferred Reporting Items as templates for our systematic review. The review process started with a search of the PubMed database to identify articles on prevalence of degenerative cervical and thoracic spinal disease. An independent reviewer assessed all articles and references and agreed on which articles should be included. To prevent selection bias during the review, abstracts from the search were numbered and pasted into a document after deleting the publication journal, author, and institution. We used the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines as templates for this systematic review. These guidelines are an evidence-based minimum set of items aimed at helping authors improve their reporting of systematic reviews and meta-analyses. The review process started with a search of the PubMed and Cochrane databases to identify articles on prevalence of degenerative cervical and thoracic spinal disease protocol. A reviewer assessed all articles and references and agreed on which articles should be included. To prevent selection bias during the review, abstracts from the search were numbered and pasted into a document after deleting the publication journal, author, and institution. The initial search included the keywords “prevalence of cervical spinal disease” and “prevalence of thoracic spinal disease” which returned 358 results.
Due to the high variation in relevant articles and anatomical locations, the search was modified to include “cervical, thoracic” which produced 309 results after duplicate articles were identified and discarded.
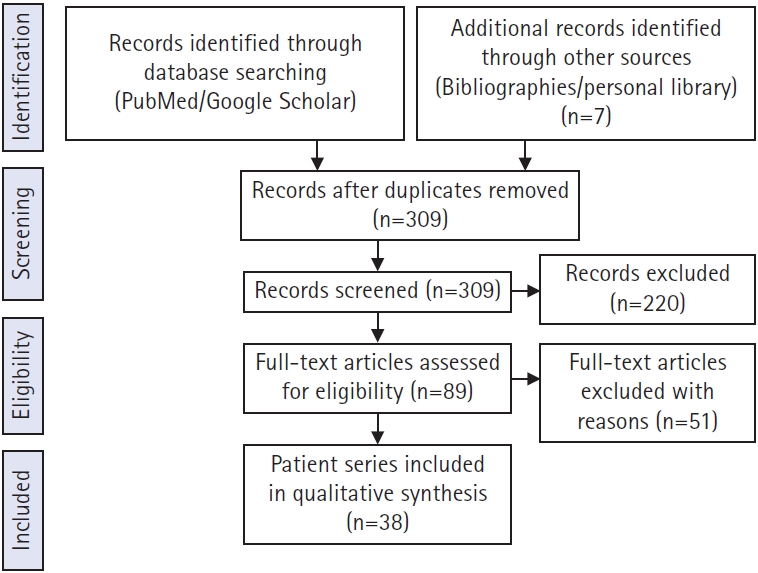
The search also included the exact surgical technique term “prevalence of symptomatic cervical and thoracic lesion” and returned 89 results published from 1980 to 2021. The exclusion criteria included reported only lumbar lesion (34 articles), deformity (17 articles), primary tumor, metastasis (5 articles), and studies not in English (2 articles). A total of 38 articles that met our inclusion criteria were identified through the search process and were analyzed (Figure 1).
There is no randomized controlled trial (RCT) that can compare prevalence of cervical and thoracic spinal disease, therefore, direct meta-analysis was not possible for both prevalences, and only narrative analysis was performed.
RESULTS
A total of 38 articles analyzing the prevalence of cervical and thoracic degenerative diseases were finally selected, among which 16 articles were on the prevalence of degenerative stenosis of the cervical spine, 10 articles on the prevalence of OPLL, and 12 articles on the thoracic lesion.
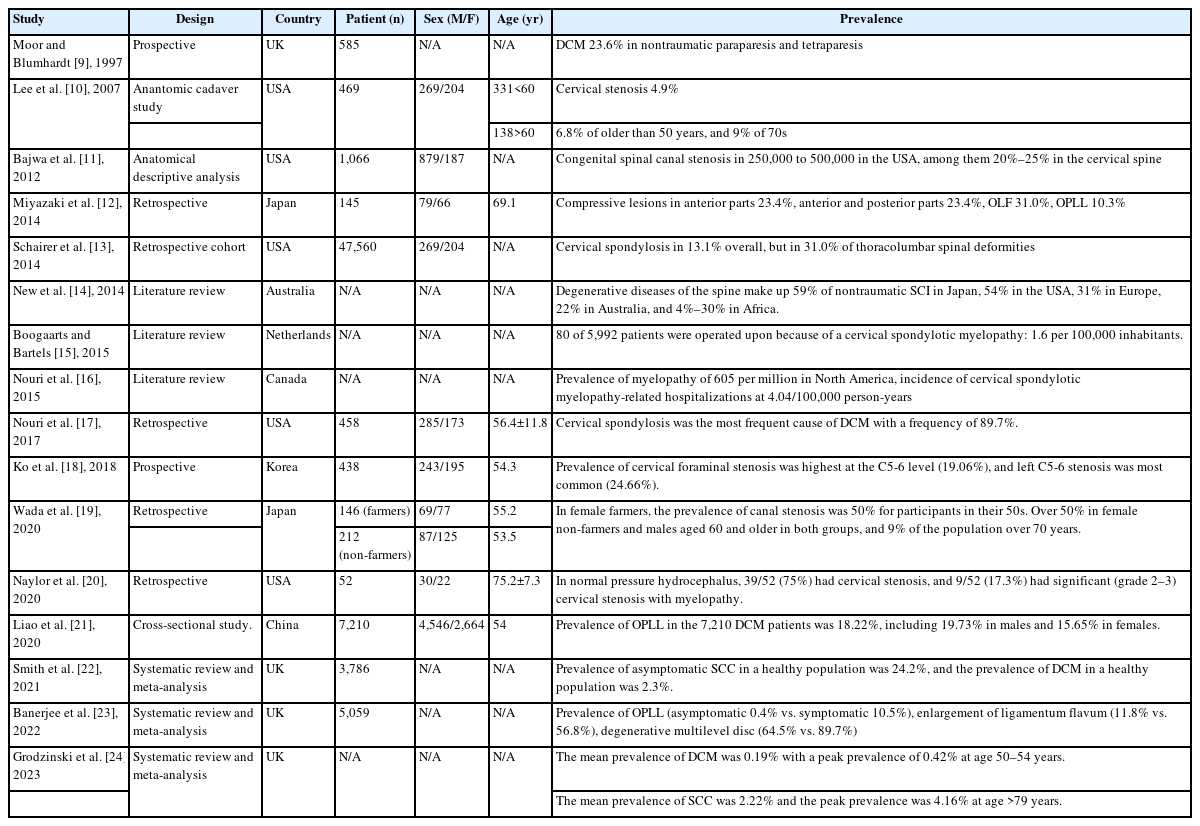
1. Prevalence of Degenerative Cervical Spondylotic Myelopathy (Table 1)
In 1997, Moore and Blumhardt [9] reported a prospective study of non-traumatic spastic paraparesis and tetraparesis in 585 patients. In this study, they found cervical spondylotic myelopathy to be the most common diagnosis (23.6%) in 585 patients admitted to a United Kingdom hospital with tetraparesis or paraparesis.
In 2007, Lee et al. [10] reported anatomic study in cadavers of prevalence of cervical spine stenosis, they estimated that cervical stenosis was present in 4.9% of the adult population, 6.8% of the population fifty years of age or older, and 9% of the population seventy years of age or older.
In 2012, Bajwa et al. [11] reported anatomical descriptive analysis of congenital cervical spinal stenosis. In this article, it has been estimated that congenital spinal canal stenosis is present in approximately 250,000 to 500,000 people in the United States, of which 20% to 25% are cervical.
In 2014, Miyazaki et al. [12] reported a retrospective study of analysis of the prevalence and distribution of cervical and thoracic compressive lesions of the spinal cord in lumbar degenerative disease. According to this study, in 145 cervical and thoracic spine, compressive lesions from the anterior parts were observed in 34 cases (23.4%). Compressive lesions from the anterior and posterior parts were observed in 34 cases (23.4%). Lesions of ossification of the ligamentum flavum were observed in 45 cases (31.0%). Lesions of ossification of the posterior longitudinal ligament were observed in 15 cases (10.3%).
In 2014, Schairer et al. [13] reported retrospective cohort study of prevalence of cervical spondylosis in patients with adult thoracolumbar spinal deformity. In this study, a total of 47,560 patients were included in this study. Cervical spondylosis occurred in 13.1% overall, but was found in 31.0% of patients with thoracolumbar spinal deformity. Similarly, thoracolumbar spinal deformity was found in 10.7% of patients overall, but was increased at 23.5% in patients with cervical spondylosis.
In 2014, New et al. [14] reported the review article of global maps of non-traumatic spinal cord injury epidemiology: towards a living data repository. In this article, the degenerative diseases of the spine make up 59% of nontraumatic spinal cord injury in Japan, 54% in the United States, 31% in Europe, 22% in Australia, and between 4% and 30% in Africa. In this same review, it was estimated that the regional incidence of nontraumatic spinal cord injury in North America, Europe, and Australia was 76, 26, and 6 per million, respectively, and that the prevalence is 1,120 per million in Canada and 2,310 per million in the Kashmir region.
In 2015, Boogaarts and Bartels [15] reported a review article of prevalence of cervical spondylotic myelopathy. In this study, surprisingly, an extensive search of the literature did not reveal exact data about the incidence or prevalence of cervical spondylotic myelopathy. The prevalence of surgically treated cervical spondylotic myelopathy was estimated as 1.6 per 100,000 inhabitants.
In 2015, Nouri et al. [16] reported a review article of degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. In this article, it can be estimated that the incidence and prevalence of nontraumatic spinal cord injury related to degenerative cervical myelopathy in the North American region is at a minimum of 41 and 605 per million, respectively.
In 2017, Nouri et al. [17] reviewed magnetic resonance (MR) images of 458 patients with degenerative cervical myelopathy (DCM) and found that cervical spondylosis was most frequent cause of degenerative cervical myelopathy with a frequency of 89.7%. Nearly 60% of spondylosis was accompanied by hypertrophy or enlargement of the ligamentum flavum (LF). Each of single-level discopathy, ossification of the posterior longitudinal ligament (OPLL), and spondylolisthesis had a prevalence with approximately 10%.
In 2018, Ko et al. [18] reported a prevalence of cervical foraminal stenosis (CFS) on computed tomography. In this study, among all 438 patients, left C5-6 stenosis was most common (24.66%), and the most severe stenosis of grade 2 was found in the left C5-6 (2.97%). The prevalence of stenosis at C4-5 was 10.50% on the right side and 13.47% on the left side; at C5-6, it was 13.47% on the right side and 24.66% on the left side; at C6-7, it was 10.96% on the right side and 12.10% on the left side. The prevalence of CFS was high in the following order: left C5-6, left C4-5 and right C5-6, and left C6-7. Overall, the incidence of CFS was greater on the left side than on the right side.
In 2020, Wada et al. [19] reported the prevalence of cervical canal stenosis in farmers. In female farmers, the rate of canal stenosis has already reached 50% among those in their 50s. The rates were over 50% in female non-farmers and males aged 60 and older in both groups. In men, the spinal canal diameter at the C4/5 level was smaller among farmers than non-farmers, and there were no significant differences at other levels. Findings of ossification of posterior longitudinal ligament existed in 5.8% of male and 3.9% of female farmers, and no significant difference in rate was found between farmers and non-farmers in both sexes.
In 2020, Naylor et al. [20] retrospectively reviewed high prevalence of cervical myelopathy in patients with idiopathic normal pressure hydrocephalus. In this study, fifty-two patients shunted for treatment of iNPH were included for analysis. 58% were male with a mean age of 75.2 years (SD 7.3 years). All patients presented with gait disturbances. 39/52 (75%) had cervical stenosis, and 9/52 (17.3%) had significant (grade 2–3) cervical stenosis with myelopathy and were subsequently treated with surgical decompression.
In 2020, Liao et al. [21] reported the overall prevalence of OPLL in the 7,210 DCM patients was 18.22%, including 19.73% in males and 15.65% in females, with a significant difference between the two groups. The prevalence of OPLL in diabetes mellitus (DM) and hypertensive patients was significantly higher than that in non-DM and normotensive patients (24.16% vs. 18.76% and 22.26% vs. 17.91%). Comparison by age and body mass index (BMI) showed that the prevalence of OPLL was the highest in the 70- to 79-year age group (21.91%) and obesity group (26.51%), respectively.
In 2021, Smith et al. [22] reported meta-analysis of the prevalence of asymptomatic and symptomatic spinal cord compression (SCC) on magnetic resonance (MR) imaging. The present search returned 1,506 publications. Following our exclusion criteria, 19 studies were included. Subgroup analysis of 3,786 individuals estimated the prevalence of asymptomatic SCC in a healthy population as 24.2% with a significantly higher prevalence of SCC in older populations compared with younger populations and American/European populations compared with Asian populations. A subgroup analysis of 1,202 individuals estimated the prevalence of DCM in a healthy population as 2.3%.
In 2022, Banerjee et al. [23] reported a review article of the prevalence of DCM-related pathologies on MR imaging. In this meta-analysis study, the search yielded a total of 1,098 studies of which 17 were included in this meta-analysis covering a total of 5,059 patients. According to this study, a prevalence of 0.4% for OPLL, 11.8% of enlargement of LF and 64.5% of degenerative multilevel disc pathology were found to be significantly lower in asymptomatic populations. On the other hand, symptomatic populations have a prevalence of 10.5% for OPLL, 56.8% for enlargement of LF and 89.7% for degenerative multilevel disc pathology.
In 2023, Grodzinski et al. [24] reported meta-analysis of most DCM remains undiagnosed, particularly amongst the elderly: modelling the prevalence of DCM in the United Kingdom. In this article, the mean prevalence of DCM across all age groups was 0.19%, with a peak prevalence of 0.42% at age 50–54 years. This contrasts with estimates from SCC data which suggest a mean prevalence of 2.22% and a peak prevalence of 4.16% at age >79 years.
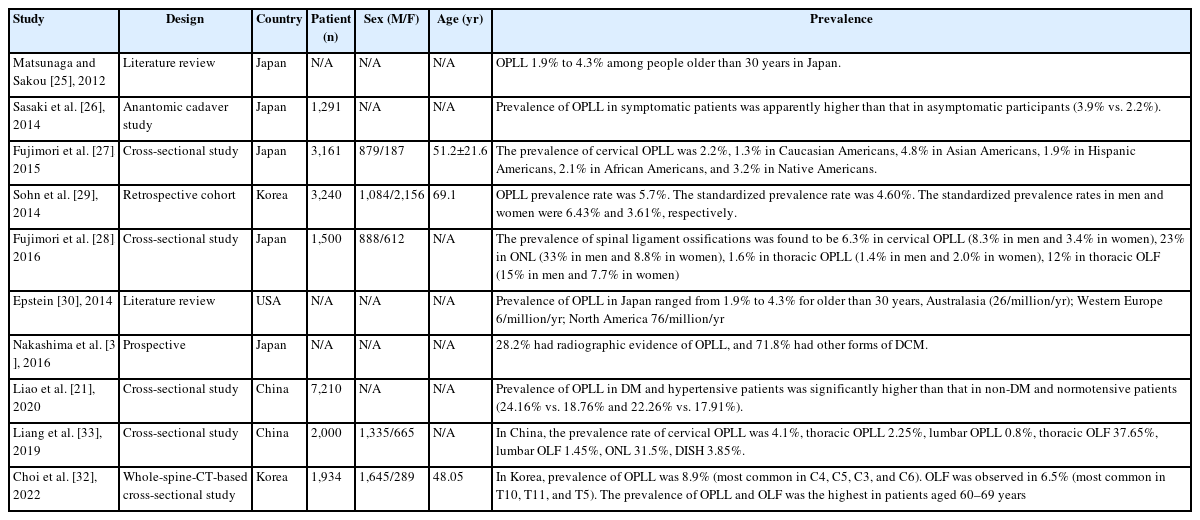
2. The Prevalence of Ossification of Posterior Longitudinal Ligament (OPLL) (Table 2)
In 2012, the prevalence of OPLL has been extensively reported. The early incidence of OPLL based on lateral radiographs was 0.1% to 1.7% in Europe, 0.1% to 1.3% in the United States, 2.1% to 3.0% in Taiwan, 0.6% to 3.6% in South Korea, and 1.9% to 4.3% in Japan [25].
Sasaki et al. [26] utilized plain X-rays to investigate the prevalence and symptoms of OPLL in 1,291 Japanese general residents, and found that the prevalence of OPLL in symptomatic patients was apparently higher than that in asymptomatic participants (3.9% vs. 2.2%).
With the development of imaging technology and equipment for the spine assessment, the recent OPLL prevalence based on CT was 1.3% to 3.2% in the United States, 5.7% in South Korea, and 6.3% in Japan given that CT has a high sensitivity to OPLL as compared to radiography [27-29] (Figure 2).
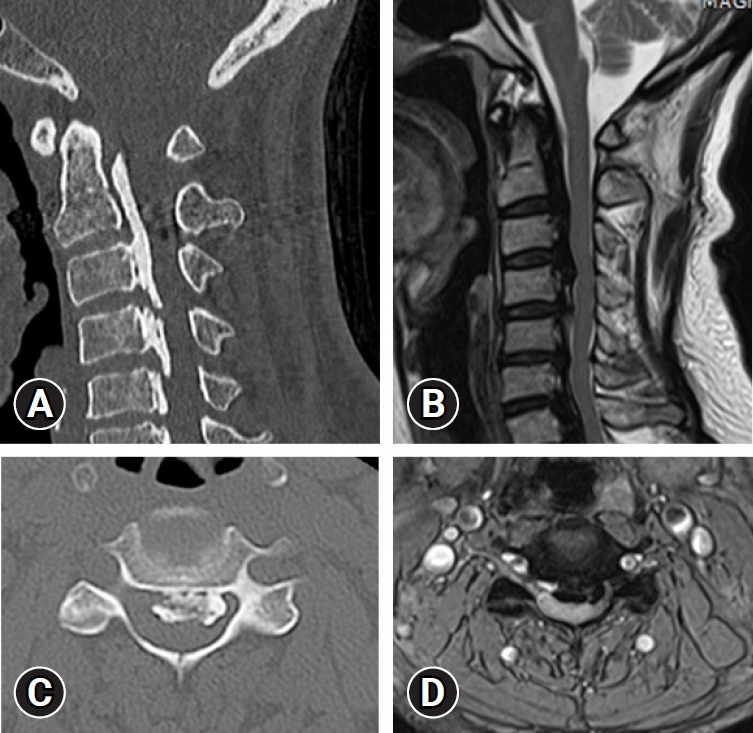
(A) Cervical reconstruction computed tomography (CT) scan shows a long strip of ossification posterior to the C2-C3 vertebral bodies and mixed configuration at C4-7. (B) Axial CT shows an ossified mass with a hole inside encroaching on the spinal canal. (C) Sagittal T2-weighted magnetic resonance imaging (MRI) shows bandlike low or no signal intensity of an ossified mass compressing the spinal cord at C2-7. (D) Axial T2- weighted MRI shows a huge ossified mass compressing the spinal cord.
Epstein [30] found that about 25% of patients treated surgically for cervical myelopathy exhibited ossification of the posterior longitudinal ligament.
In 2016, Nakashima et al. [31] studied 479 patients with symptomatic DCM based on AO spine cervical spondylotic myelopathy (CSM)-international study database, and found that the overall prevalence of OPLL in patients with DCM was 28.18% and 35.33% in the Asian and Pacific populations, 18.70% in North American populations, and 31.75% in European populations.
Liao et al. [21] reported a cross-sectional study of the prevalence of OPLL in patients with DCM: cervical spine 3D CT observations in 7,210 cases. In this article, the results showed that the prevalence rate of OPLL in patients with DCM was 18.22%, which is much higher than that in the general Asian population as reported in previous studies, indicating that OPLL commonly coexists with degenerative spondylosis in the cervical spine.
In 2016, Fujimori et al. [28] reported prevalence, concomitance, and distribution of ossification of the spinal ligaments: results of whole spine CT scans in 1,500 Japanese patients. In this study, the prevalence of spinal ligament ossifications was found to be 6.3% in cervical OPLL (8.3% in men and 3.4% in women), 23% in ossification of nuchal ligament (ONL) (33% in men and 8.8% in women), 1.6% in thoracic OPLL (1.4% in men and 2.0% in women), 12% in thoracic OLF (15% in men and 7.7% in women), 37% in thoracolumbar ossification of anterior longitudinal ligament (OALL) (45% in men and 26% in women), and 2% in diffuse idiopathic skeletal hyperostosis (DISH) (16% in men and 6.2% in women). Thirteen percent of patients with cervical OPLL had thoracic OPLL, 34% of cervical OPLL had thoracic OLF, 45% of cervical OPLL had ONL, and 36% of cervical OPLL had DISH. The most common level was C5 for cervical OPLL, T1/2 for thoracic OPLL, T11 for thoracic OLF, and T8/9 for OALL.
In 2022, Choi et al. [32] reported in 2020 prevalence, distribution, and concomitance of whole-spine OPLL and OLF in South Koreans. A total of 1,934 adults (1,645 men, 289 women) were included. The mean age was 48.05 years (range, 28–86 years). Among the 1,934 patients, 173 had OPLL (8.9%). The most commonly involved cervical vertebra levels arranged according to frequency were C4, C5, C3, and C6. OLF was observed in 125 patients (6.5%). The most commonly involved thoracic levels were T10, T11, and T5. The prevalence of OPLL and OLF was the highest in patients aged 60–69 years. Among the C-OPLL patients, 15.1% had T-OPLL, 5.0% had L-OPLL, and 25.8% had T-OLF.
In 2019, Liang et al. [33] reported epidemiology of ossification of the spinal ligaments and associated factors in the Chinese population: a cross-sectional study. The prevalence rate of cervical OPLL was 4.1%, thoracic OPLL 2.25%, lumbar OPLL 0.8%, thoracic OLF 37.65%, lumbar OLF 1.45%, ONL 31.5%, DISH 3.85%. The most commonly involved level was C5 for C-OPLL, T1 for T-OPLL, T10 for T-OLF, and T8/9 for OALL. 21% of subjects with C-OPLL had T-OPLL, 44% of C-OPLL had T-OLF, 38% of T-OPLL had C-OPLL, 53% of T-OPLL had T-OLF, 44% of L-OPLL had T-OPLL, and 56% of L-OPLL had T-OLF (Figure 3).
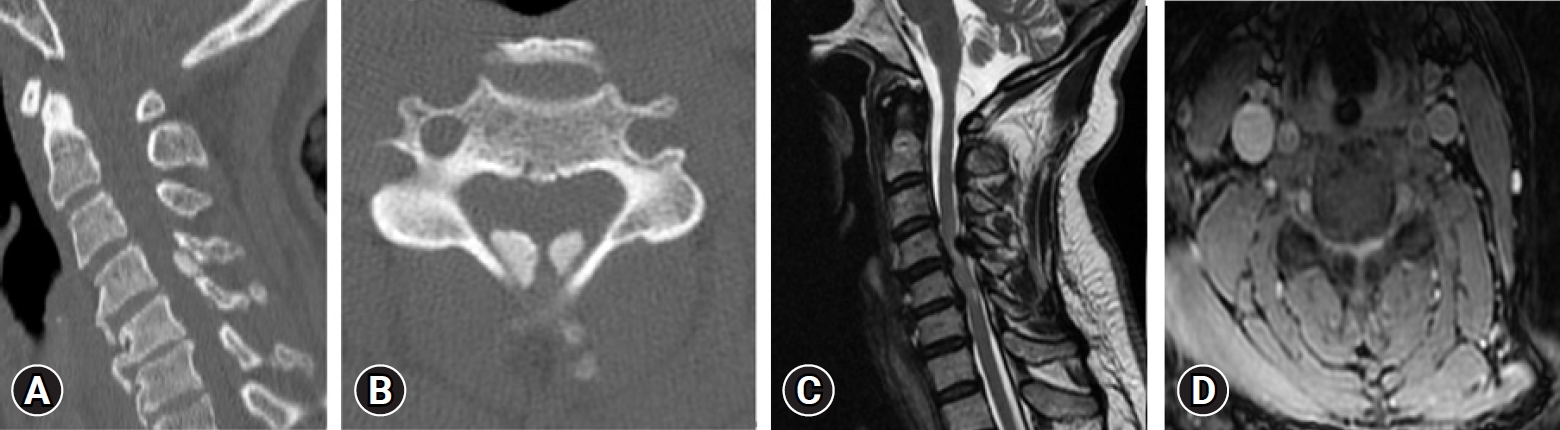
(A) Sagittal computed tomography (CT) scans demonstrating the local ossified ligamentum flavum extending on C4-5.(B) Axial CT scan at the level of C4-5 demonstrating significant posteromedial compression in the spinal canal. (C) Abnormally high T2 signal of the cervical cord opposite the C4-5 level, with the cervical cord being compressed between the right paracentral disc protrusion and abnormal bilateral symmetrical posterior extradural lesions with low signal on all pulse sequences. (D) Abnormally high T2 signal of the cervical cord opposite the C4-5 level, with the cervical cord being compressed between right paracentral disc protrusion and abnormal bilateral symmetrical posterior extradural lesions with low signal on all pulse sequences.
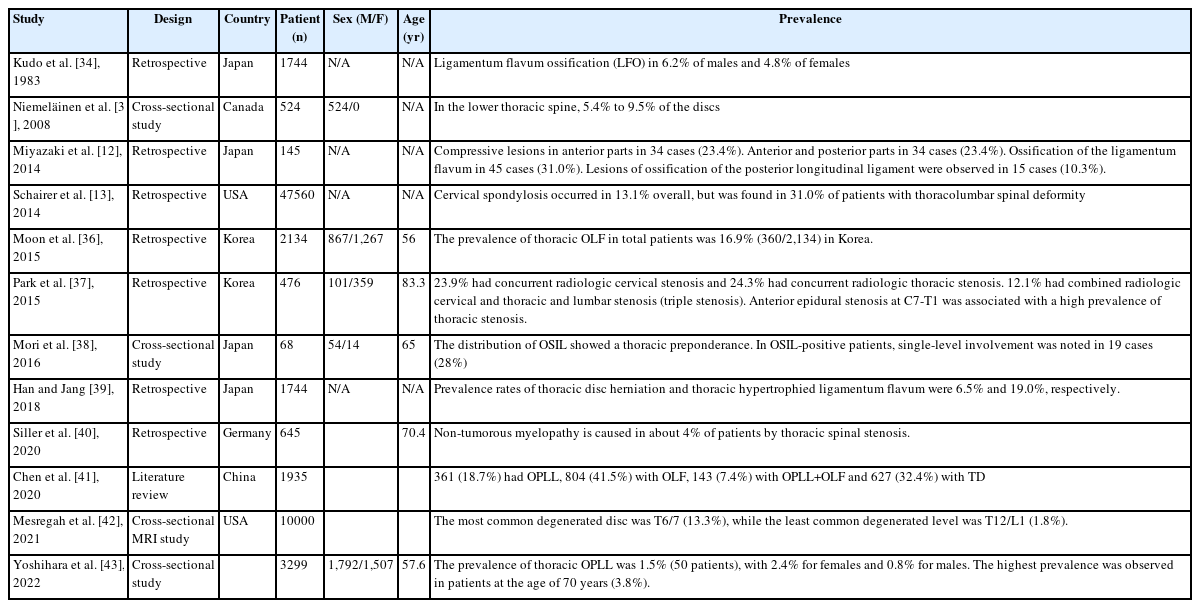
3. Prevalence of Thoracic Degenerative Spinal Disease (Table 3)
In 1983, Kudo et al. [34] reported a long-term follow-up investigation of a fixed sample of ossification of thoracic ligamenta flava. In this article, review of 1,744 consecutive lateral chest radiographs identified ligamentum flavum ossification (LFO) in 6.2% of males and 4.8% of females. LFO occurred mainly at the intervertebral segments from T9-T10 through T12-L1. Most prevalent was the hook-shaped LFO, protruding inferiorly from the inferior facets into the projections of the intervertebral foramina. Though LFO can cause severe neurologic symptoms, none of the affected persons in this study reported such symptoms. LFO was first visualized radiographically when the subjects were 20–40 years old, and it may be a physiologic condition.
In 2008, Niemeläinen et al. [35] reported a cross-sectional study of thoracic magnetic resonance image (MRI) findings. In this study, in the lower thoracic spine, 5.4% to 9.5% of the discs, depending on level, were qualitatively assessed as moderately to severely narrowed. Anterior bulging was more common than posterior, which was relatively rare and mild when present. Signal was lower in the midthoracic than lower discs. At least 1 moderate or severe vertebral deformity was found in 6.1% of the subjects, suggesting fracture, and hemangiomas were identified in 2.3% of subjects.
In 2014, Miyazaki et al. [12] reported retrospective cohort study of prevalence and distribution of cervical and thoracic compressive lesions of the spinal cord in lumbar degenerative disease. In this article, the DISH was present in 25.6% of patients (72/281). The prevalence of DISH in the 41–49, 50–59, 60–69, 70–79, and ≥80 year age groups was 8.3% (2/24), 9.8% (5/51), 16.0% (12/75), 49.5% (48/97), and 33.3% (4/12), respectively; the prevalence increased with age. The average number of fused vertebral bodies was 7.5. More than 80% of DISH was located from T7 to T11, and more than 95% of DISH was located at T9/10. Patients with DISH were significantly older (71.1 years vs. 60.9 years), and men were more likely to have DISH than women.
In 2014, Schairer et al. [13] reported a retrospective cohort study of the increased prevalence of cervical spondylosis in patients with adult thoracolumbar spinal deformity. In this article, a total of 47,560 patients were included in this study. Cervical spondylosis occurred in 13.1% overall, but was found in 31.0% of patients with thoracolumbar spinal deformity. Similarly, thoracolumbar spinal deformity was found in 10.7% of patients overall, but was increased at 23.5% in patients with cervical spondylosis.
Moon et al. [36] reported prevalence, distribution, and significance of incidental thoracic OLF in Korean patients with back or leg pain. In this study, the prevalence of thoracic OLF in total patients was 16.9% (360/2,134). The prevalence tended to increase with aging and was higher in women than in men. The lower thoracic segment of T10-11 was the most frequently affected segment. Of the 360 patients with OLF, 31.9% had coexisting herniated thoracic discs at the same level. Approximately 74% of the patients with OLF had coexisting lumbar and cervical disease. Nine (2.5%) of 360 OLF patients underwent surgery for thoracic lesion.
Park et al. [37] reported among the 460 patients with lumbar stenosis, 110 (23.9%) had concurrent radiologic cervical stenosis and 112 (24.3%) had concurrent radiologic thoracic stenosis. Fifty-six patients (12.1%) had combined radiologic cervical and thoracic stenosis in addition to their symptomatic lumbar stenosis (triple stenosis). Anterior epidural stenosis at C7-T1 was associated with a high prevalence of thoracic stenosis.
Mori et al. [38] reported prevalence and distribution of ossification of the supra/interspinous ligaments (OSIL) in symptomatic patients with cervical ossification of the posterior longitudinal ligament of the spine. In this study, a total of 234 patients with a mean age of 65 years was recruited. The CT-based evidence of OSIL was noted in 68 (54 males and 14 females) patients (29%). The distribution of OSIL showed a significant thoracic preponderance. In OSIL-positive patients, single-level involvement was noted in 19 cases (28%), whereas 49 cases (72%) presented multi-level involvement. We found a significant positive correlation between the OP-index gradevand OSI-index. ONL was noted at a significantly higher rate in OSIL-positive patients compared to negative patients
Han and Jang [39] reported prevalence and distribution of incidental thoracic disc herniation (TDH), and thoracic hypertrophied ligamentum flavum. The prevalence rates of TDH and thoracic HLFS in all patients were 6.5% (145/2,212) and 19.0% (421/2,212), respectively. The prevalence of TDH was demonstrated as a relatively even distribution across age groups higher in male participants (8.0%) than in female participants, and more frequent in patients with lumbar surgical lesions (8.2%) than without surgical lesions. Whereas, the prevalence of thoracic HLFS tended to increase with age, was higher in female participants (21.6%) than in male participants, and had no association with presence of lumbar surgical lesions. The most frequently involved segments of TDH and HLFS were T8/9 and T10/11, respectively. Six of 145 patients with TDH and 15 of 421 patients with HLFS underwent surgery.
In 2020, Siller et al. [40] reported the retrospective study of surgery of degenerative thoracic spinal stenosis-long-term outcome with quality-of-life after posterior decompression via a uni- or bilateral approach. In this study, From 645 patients with surgery for degenerative spondylotic myelopathy within 6 years, 28 patients (4.3%) suffered from thoracic spinal stenosis. Median age was 70.4 years with a slight predominance of the female sex. The most frequent symptoms (mean duration 7.6 months) were ataxia (61%) and sensory changes (50%).
In 2020, Chen et al. [41] reported the review article of the prevalence and clinical characteristics of thoracic spinal stenosis: a systematic review. In this study, a total of 129 studies including 1,935 subjects were selected, of which 361 (18.7%) were diagnosed with OPLL, 804 (41.5%) with OLF, 143 (7.4%) with OPLL+OLF and 627 (32.4%) with TDH. Most reports were from China, Japan and USA. Thoracic OPLL occurred mostly at the middle-thoracic spine (43.4%), while OLF predominately occurred at the lower-thoracic spine (63.1%). TDH was mainly localized in the middle (46.0%) and lower-thoracic (50.3%) spine. Thirty-two studies involving 524 patients described tandem spinal stenosis, of which 52.1% had accompanying cervical diseases and 35.9% lumbar diseases.
In 2021, Mesregah et al. [42] reported a cross-sectional MRI study of trends and patterns of thoracic intervertebral disc degeneration in symptomatic subjects. In this study, the total grade of IVD degeneration and the number of degenerated levels increased with increasing age. The most common degenerated level was T6/7 (13.3%), while the least common degenerated level was T12/L1 (1.8%). The most common grades were grade I in group 1 (60.5%), grade II in groups 2 (39%) and 3 (37.3%), and grade III in groups 4 (42.5%) and 5 (44.6%).
Yoshihara et al. [43] reported prevalence and characteristics of thoracic ossification of the posterior longitudinal ligament in 3,299 Black patients. In this study, the prevalence of T-OPLL was 1.5% (50 patients), with 2.4% for females and 0.8% for males. The highest prevalence was observed in patients at the age of 70 years (3.8%). Thickness of T-OPLL was between 2 and 3mm in 46% (23/50) of the patients, and the largest thickness was 6.1 mm. T-OPLL was significantly associated with female sex and the presence of DM.
DISCUSSION
DCM without trauma is the most common cause of spinal cord injury in the elderly population [44]. This DCM largely includes CSM, OPLL, OLF, and degenerative disc disease (DDD).
Tissue degeneration anywhere in the body progresses primarily as a function of the intensity of use over time. However, musculoskeletal structures that bear significant structural loads may be subject to accelerated deterioration.
In the cervical spine, these degenerative changes can be divided into spinal (or osteoarthritic) and non-osteoarthritis changes, with additional subtype classifications. However, although these pathological changes are isolated as separate clinical entities, there are loose differences between them in practical terms, as they are highly interrelated and often appear simultaneously [16].
Ultimately, a major integrative problem is the propensity of degenerative changes to cause spinal canal stenosis, cause spinal cord compression, and eventually lead to disability due to the development of myelopathy.
Pathophysiologically symptomatic DCM can result from static compression of the spinal cord, misalignment of the spine leading to changes in spinal tension and vascular supply, and repetitive dynamic injuries resulting from segmental hypermobility. In the latter case, it has also been recognized that unstable spinal segments can be responsible for chronic repeated microtrauma to the spinal cord that is not large enough to be recognized as traumatic spinal cord injury (SCI) [16]. Additionally, people with spinal stenosis who have experienced minor trauma to the neck have a significantly higher risk of developing myelopathy or worsening an existing myelopathy [45]. Thus, some degree of trauma is likely to contribute to the natural history of DCM development.
One possible way to estimate the incidence and prevalence of DCM is to look at the reported rates of SCI, which are commonly classified as traumatic and non-traumatic forms. DCM is included in these estimates because it represents a non-traumatic form of SCI.
New et al. [14] found that degenerative spinal diseases account for 59% of non-traumatic form of spinal cord injury in Japan, 54% in the United States, 31% in Europe, 22% in Australia, and 4% to 30% in Africa. In this review article, the regional incidence of non-traumatic form of spinal cord injury was estimated to be 76, 26, and 6 per million in North America, Europe, and Australia, respectively, and the prevalence was estimated to be 1,120 in Canada and 2,310 in the Kashimr region, India. From these figures, the incidence and prevalence of DCM-associated non-traumatic form of spinal cord injury in North America can be estimated to be at least 41 and 605 per million, respectively.
However, these data include the fact that they generally do not include patients with mild symptoms, but only patients with documented paraplegia and quadriplegia due to severe non-traumatic form of SCI. The perception that many patients with myelopathy will have a milder clinical picture indicates that the aforementioned figures are significantly underestimated and likely represent only patients at the severe end of the disease spectrum.
Investigations into the underlying genetic basis for OPLL have been conducted by many researchers. The fact that OPLL is much more frequent in Asian than Caucasian populations supports the presence of a genetic etiological component. In fact, in a nationwide survey of OPLL patients in Japan, it was found that 24% of second-degree relatives and 30% of siblings of OPLL patients had radiographically detectable OPLL [8]. Based on the pathological distribution, OPLL has been classified into four subtypes. Localized, segmental; continuous; mixed with segmental are considered the most common types. Kudo et al. [46] investigated at the genetic differences between these subtypes and proposed two categories for OPLL: continuous (including continuous and mixed) and segmental (including segmental and circumscribed). They concluded that cells from the OPLL contiguous group had a higher osteogenic differentiation potency than cells from the segmental group, and that different genetic backgrounds existed between groups.
Whether there is a genetic susceptibility to future OPLL and cervical degenerative diseases such as ossification of the flavum ligament, whether such susceptibility is genetically distinct from OPLL, and whether there is a generalized spinal ligament ossification condition such as diffuse idiopathic skeletal hyperostosis. It is clear that further research is needed.
CONCLUSION
Various forms of degenerative cervical spinal disease, including stenosis, which can compress cervical spinal cord and nerve, appear to be very common. Therefore, radiographic findings of cervical spinal stenotic lesions must be correlated with clinical symptoms before making treatment decisions.
DCM is an overarching term used to describe the various degenerative conditions of the cervical spine that result in myelopathy, including CSM, DDD, OPLL, and OLF. Pathophysiologically, symptomatic DCMs result from static compression of the spinal cord, spinal malalignment leading to altered cord tension and vascular supply, as well as dynamic injury mechanisms.
It is important to accurately identify the prevalence of various types of cervical spinal cord and nerve compression disorders and to predict the possibility of neurological symptoms due to progressive degenerative diseases such as ossification in the future. Therefore, accurate diagnosis using CT and MRI requires treatment planning and patient education.
Notes
Ethical statements
This study was exempted from IRB review as it was a study using information disclosed to the general public or a study that did not collect and record personally identifiable information.
Conflict of interest
Pius Kim is the Editor of the Journal of Minimally Invasive Spine Surgery and Technique and was not involved in the review process of this article. All authors have no other potential conflicts of interest to declare relevant to this article.