Unilateral Biportal Endoscopic Decompression for Thoracic Spinal Stenosis
Article information
Abstract
In conventional thoracic laminectomy, postoperative complications such as dural tears, cerebrospinal fluid leakage, postoperative infection, and iatrogenic spinal cord injury are relatively common. To address these issues, unilateral biportal endoscopy (UBE) techniques for thoracic laminectomy have been developed and published, demonstrating various advantages over conventional thoracic laminectomy and satisfactory clinical results. In comparison to conventional thoracic laminectomy, the UBE decompression technique with the unilateral approach and bilateral decompression (ULBD) appears to be safe and effective for treating thoracic ossified ligamentum flavum (OLF) or thoracic spinal stenosis. Thoracic ULBD by UBE preserves the paraspinal muscle and ligament that would otherwise be resected during the conventional posterior approach, and it has the added benefit of reducing back pain and muscle atrophy. This manuscript covers the technical aspects of UBE implementation using thoracic ULBD in patients with thoracic OLF or thoracic spinal stenosis, the indications and benefits of this procedure, and tips for preventing complications. With a review of previously published articles on thoracic laminectomy by UBE, we also summarize whether the procedure is safe and can prevent cord injuries.
INTRODUCTION
Traditional thoracic laminectomy has been considered the gold standard for treating thoracic ossified ligamentum flavum (OLF) or thoracic spinal stenosis [1-3]; however, it has several drawbacks, including postoperative back pain and paraspinal muscle atrophy caused by posterior musculoligamentous and facet joint injury [4]. As a result, fusion surgery may be required in some cases to prevent postoperative instability [5]. In a conventional thoracic laminectomy, postoperative complications such as dural tears, cerebrospinal fluid leakage, postoperative infection, and iatrogenic spinal cord injury are relatively common [6,7]. The clinical outcomes of a conventional technique for thoracic OLF or thoracic spinal stenosis are frequently unsatisfactory and comorbid [3,8].
To address these issues, unilateral biportal endoscopy (UBE) techniques for thoracic laminectomy have been developed and published by some studies, demonstrating various advantages over conventional thoracic laminectomy and reporting competent clinical results [9,10]. For lumbar spinal stenosis, the concept of unilateral laminectomy for bilateral decompression (ULBD) has been successfully managed [11]. The ULBD using UBE in the treatment of thoracic OLF or thoracic spinal stenosis is also being investigated. In comparison to conventional thoracic laminectomy, the UBE technique for thoracic pathology may provide appropriate decompression with technical advantages and fewer complications, such as minimal musculoligamentous injury or facet joint destruction and minimal spinal cord manipulation [9,10]. Furthermore, the endoscope and surgical instruments can be moved independently, making the operation safer and more effective than other endoscopic techniques [12].
Present manuscript has covered technical aspect of UBE decompression of thoracic OLF and thoracic spinal canal stenosis with its indications, advantages and tips to prevent complications. With a review of previously published articles on thoracic laminectomy by UBE, we also summarized whether the procedure is safe and can prevent cord injuries.
INDICATIONS
When conservative treatment fails or the patient's neurological condition worsens, surgical treatment of thoracic ULBD by UBE is recommended. The following are the indications and contraindications for thoracic ULBD by UBE: 1) Thoracic spinal stenosis; 2) OLF; 3) Synovial cysts. Contraindications of thoracic ULBD by UBE are as follows:1) Central disc herniation; 2) Severe ossified posterior longitudinal ligament (OPLL); 3) Spinal tumor; 4) Instability of the spinal column; 5) High-grade deformity; 6) Fused/tuberous type OLF, Severe thoracic stenosis or severe dural ossification; because of risk and technical challenge.
SURGICAL PROCEDURE
1. Anesthesia and Position
Patient is placed prone on a radiolucent table after general anesthesia and intraoperative neurophysiological monitoring. Right-handed surgeons prefer the left-side approach because it facilitates bone work. It is recommended that the working portal for surgical instrument manipulation be used with the dominant hand. The scopic portal for endoscopic viewing, on the other hand, is used with the non-dominant hand.
2. Surgical Instruments
Most of the surgical instruments used in thoracic decompression by UBE are comparable to those used in other UBE procedures. This technique requires a diamond drill and a 1 mm Kerrison rongeur. A 0-degree scope is typically utilized, but a 30-degree scope is necessary to approach a centrally located thoracic disc herniation. Hook radiofrequency probe is used for coagulation of focal epidural vessels. Using pressure pump systems is not preferred as saline can be sufficiently infused by gravity, which is enough to achieve a clear view while minimizing epidural bleeding. The proper height of the fluid back is about 40–60 cm from the patient’s back.
3. Skin Markings and Creating Portals
After positioning the patient, the inferior endplate line of the upper vertebral body is paralleled using C-arm fluoroscopic guidance. The docking point, the lower part of the cranial lamina, is confirmed using fluoroscopy’s anteroposterior (AP) view. Two skin incisions, approximately 3 cm apart, are made on the medial margin of the proximal and distal pedicle, centered at the docking point (Figure 1A). A caudal skin incision is made for the working portal. And a cranial incision is made for the scope portal. A series of dilators are sequentially inserted via the working portal under fluoroscopic guidance, and an endoscope sheath is inserted through the scope portal to the docking point. The dilator tip and endoscopic sheath are triangulated over the docking point, and AP and lateral fluoroscopy are used to ensure proper positioning (Figure 1B).
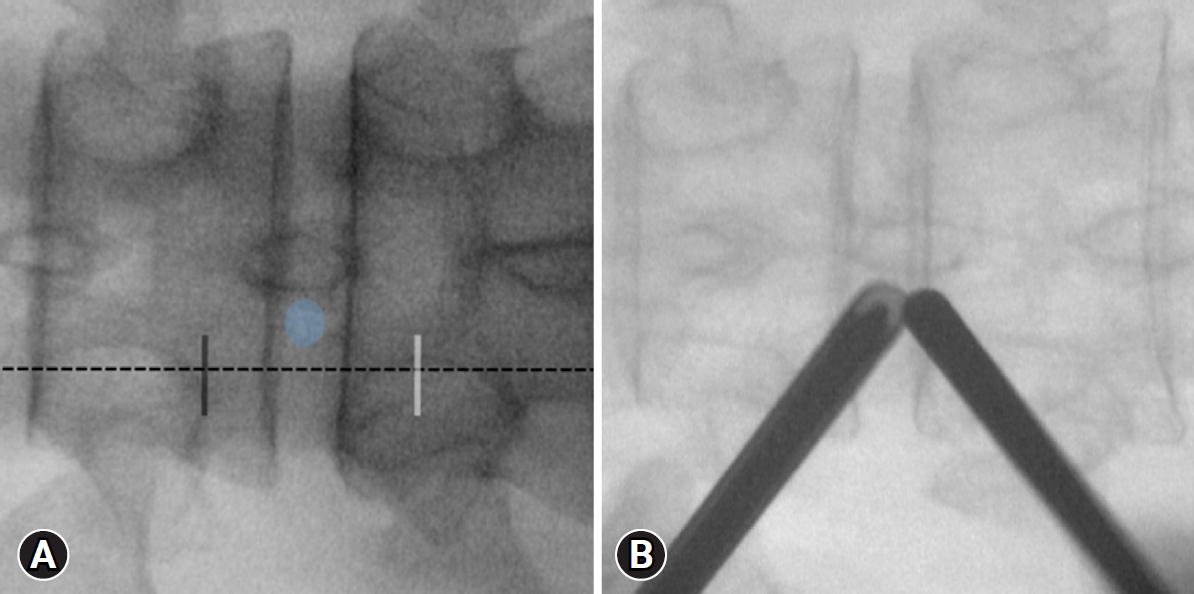
Fluoroscopic anteroposterior (AP) view of the docking point and skin incision at T10-T11 levels. The docking point (blue circle) is the lower part of the cranial lamina. Two skin incisions (working portal: white line; scopic portal: black line) are made approximately 3 cm apart, with the center as the lower part of the cranial lamina at the midline of the proximal and distal pedicles (dotted line) (A). Triangulation of the dilator and the endoscopic sheath above the docking point on a fluoroscopic AP view (B).
4. Bone Working
The lower part of the cranial lamina and interlaminar space are identified after soft tissue is removed with a radiofrequency (RF) probe (Figure 2A). Subsequently, cranial lamina is resected with the help of burr or kerrisons ronguer from caudal to cranial direction to expose ligamentum flavum (LF). At this point, it is critical to avoid burr or Kerrison rongeur compression of the LF. The midline gap of the LF, which serves as an anatomical landmark for midline orientation (Figure 2B). Cranial bone work is performed up to the anatomical landmark of the cranial end of the LF. To prevent cord injury while removing the lamina, the LF is used as a defender until the bone work is finished.
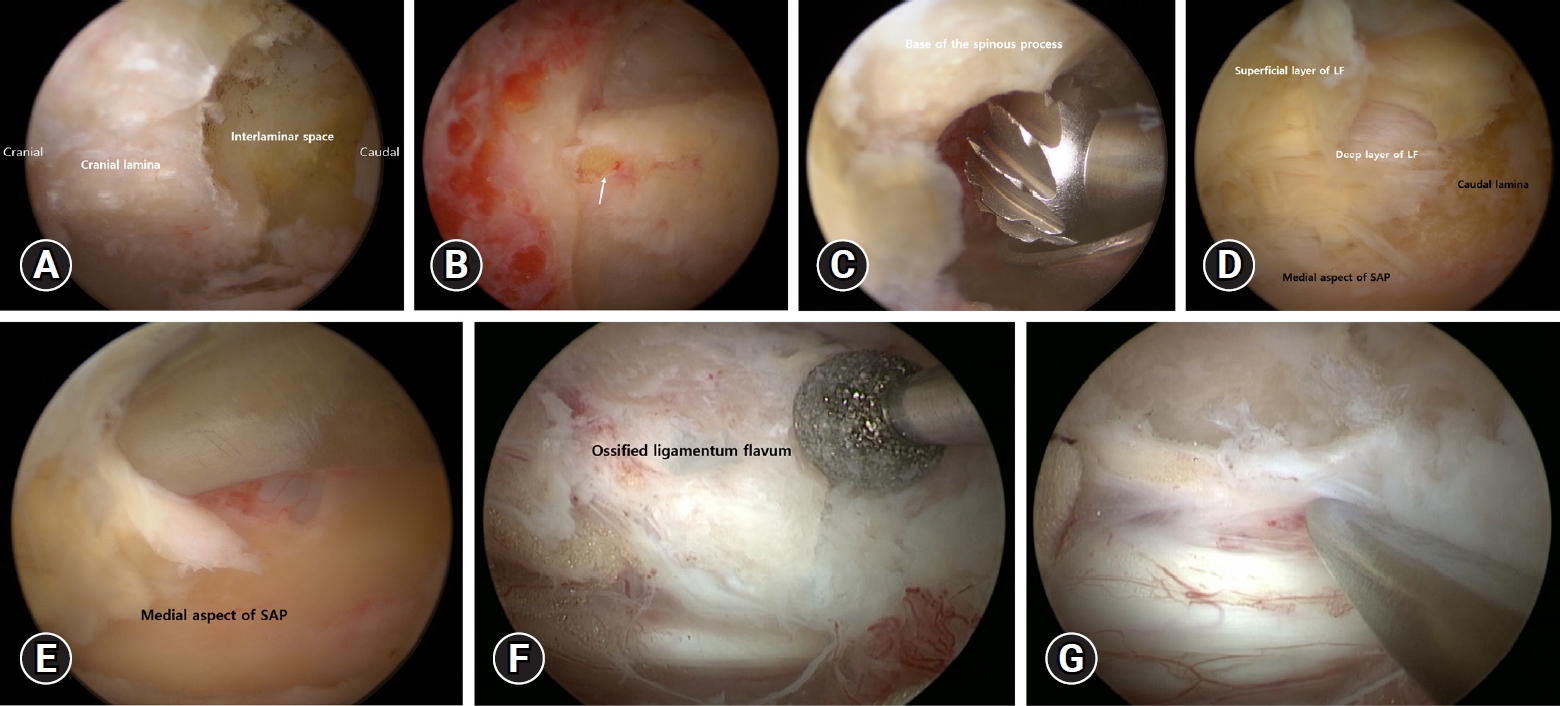
Serial sequence endoscopic images of thoracic ULBD by UBE. The surgical anatomy is first noted at the lower part of the cranial lamina, and the interlaminar space (A). Anatomical landmark for midline orientation. The white arrow indicates the midline gap of the ligamentum flavum (LF) (B). The base of the spinous process should be removed to provide enough space for safe decompression (C). Exposure of the upper portion of the caudal lamina and medial margin of the superior articular process after removal of the superficial layer of the LF (D). Removal of the paper-thin, translucent medial part of superior articular process using double-ended elevator (E). The OLF is ground into a thin form using a diamond drill (F). The thinned OLF can be detached from the dural sac using a double-ended elevator (G). ULBD: unilateral approach and bilateral decompression, UBE: unilateral biportal endoscopy; OLF: ossified ligamentum flavum.
When performing a contralateral bone working via a sublaminar approach, contralateral lamina and the base of the spinous process is drilled to allow enough space on the contralateral side (because the base of the spinous process interrupts the movement of the surgical instruments and the endoscope) (Figure 2C). The medial part of the facet joint is partially removed after a wide laminectomy to decompress both sides. The lateral end of the laminectomy overlaps the medial aspect of the facet joint, which is preserved to prevent instability.
5. Removal of Ligamentum Flavum
Following bone work, a double-ended elevator and pituitary forceps are used to remove the superficial layer of LF from the caudal lamina (Figure 2D). Following that, a landmark for lateral decompression is identified as the junction between the caudal lamina and the medial aspect of the superior articular process (SAP) (Figure 2D). To prevent the Kerrison rongeur from compressing the spinal cord beneath the bony structures, the caudal lamina and the medial part of the SAP are ground thin with a diamond drill. The LF is carefully separated after thinning with a 1 mm Kerrison rongeur or double-ended elevator that continues along the medial aspect of the SAP, allowing En-block removal of the deep layer of LF (Figure 2E). The procedure for removing the contralateral LF is the same as described above.
In the case of OLF, the OLF is identified by removing the LF’s superficial layer. With the use of diamond drill OLF is thinned out as much as possible (Figure 2F). The remaining thinned OLF was separated from the dura with a double-ended elevator and carefully removed one at a time with small-sized pituitary forceps (Figure 2G). If the dura mater and OLF are not separated by adhesions or dural ossification, the calcified portion of the OLF is thinned, leaving a thinned OLF. The floating method is known to prevent dural tear and cord injury. The decompression is completed at the medial margins of the pedicle and lateral margin of the thecal sac. Finally, a free-floating dura indicates adequate decompression under endoscopic guidance.
6. The Final Checking Point
The adhesion of the OLF to a dura sac, which can lead to the dura tear, a complication of a small dura tear, can be managed by a applying a fibrin collagen patch (TachoSil) and bed resting for 7 days. If the dura tear is large enough that the fibrin collagen patch cannot cover it, the dural tear can be repaired directly through dural suturing or microscopic surgery. To address this complication, preoperative CT and MRI should be checked to look for dural ossification within the OLF.
To prevent postoperative hematoma, a Jackson–Pratt surgical drain (100 mL) is inserted via the working portal after the operation. Inserting the Jackson–Pratt drain deeply should be done with caution because the tip of the drain can cause cord injury. Postoperative MRI should be checked at the second day after surgery. Jackson–Pratt surgical drain is removed 1 or 2 days postoperatively.
RESULTS
1. Case 1: Ossified Ligamentum Flavum
A 57-year-old man presented with spastic paraplegia, Nurick’s grade 4 that had been present for 12 months. Preoperative MRI and CT showed bilateral OLF compressing the cord at the T4-T5 level (Figure 3A–C). ULBD by UBE was performed on the left side at the T4-T5 level. The operation time was about 65 minutes. The OLF was removed, and the thecal sac was decompressed completely. Postoperative MRI and CT scans confirmed complete decompression (Figure 3D, E). Following a 4-month follow-up after, both lower extremity weakness was restored to grade 5. His neurologic symptoms were improved to Nurick’s grade 0.
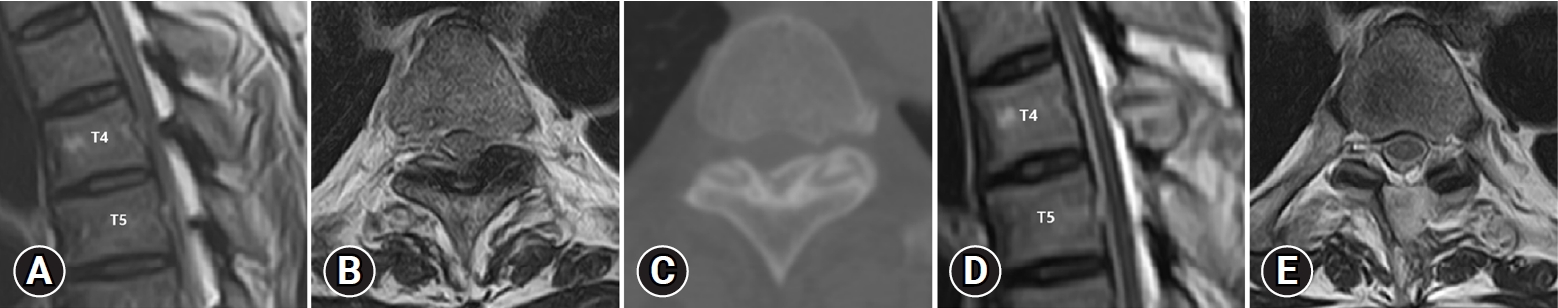
Images of a 57-year-old man with ossified ligamentum flavum at the T4-T5 level. Preoperative MRI and CT shows ossified ligamentum flavum at the T4-T5 level (MRI sagittal: A, axial: B, CT axial: C). Postoperative axial T2-weighted MRI shows complete removal of ossified ligamentum flavum (sagittal: D, axial: E). MRI: magnetic resonance imaging, CT: computed tomography.
2. Case 2: Thoracic Spinal Stenosis
A 71-year-old man presented with bilateral lower extremity weakness with compressive myelopathy from thoracic spinal stenosis at T11-T12 since 9 months. For three months, he received conservative treatment. His symptoms, however, did not improve and worsened. His Nurick’s grade was 3. Preoperative MRI scan showed T11-T12 thoracic spinal stenosis (Figure 4A, B). At T11–T12, bilateral hypertrophic LF compressed the spinal cord. The operation time was about 55 minutes. MRI scans after surgery revealed adequate spinal cord decompression at the T11-T12 level (Figure 4C, D). The neurologic symptoms significantly were improved to Nurick’s grade 0.
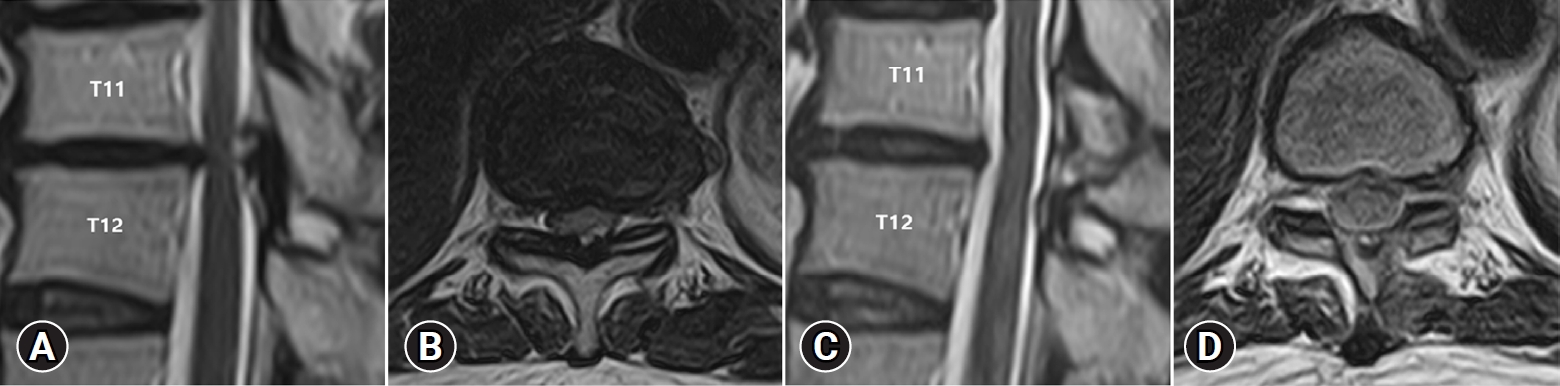
Images of a 71-year-old man with thoracic spinal stenosis at the T11-T12 level. Preoperative MRI shows thoracic spinal stenosis and compression of the cord at the T11-T12 level (MRI sagittal: A, axial: B). Postoperative axial T2-weighted MRI shows sufficient decompression of the thoracic spinal stenosis (MRI sagittal: C, axial: D). MRI: magnetic resonance imaging.
DISCUSSSION
Due to a low canal-to-spinal cord ratio, thoracic kyphosis pushing the spinal cord anteriorly, and poor blood supply in the watershed area, the thoracic spinal cord is vulnerable. Excessive manipulation of the thoracic spinal cord with surgical instruments increases the risk of neurological deterioration; thus, thoracic decompression should be performed with caution to avoid unintended spinal cord injury [13]. As a result, the goal of thoracic decompression is to achieve adequate decompression with minimal spinal cord manipulation while avoiding paraspinal muscle/facet injury.
The thoracic ULBD by UBE has several advantages over conventional laminectomy for OLF or thoracic spinal stenosis: (1) The combination of an angled field of view and freedom of movement allows enough space and minimal spinal cord manipulation [12]. This can aid in adequate decompression and improve clinical outcomes while avoiding the complications associated with conventional thoracic laminectomy. (2) While operating under continuous saline irrigation, UBE provides a familiar operative view and high magnification/clearing availability [10]. Furthermore, this technique can achieve precise OLF removal while lowering the risk of dural tear or cord injury, which is identified under endoscopic view. (3) When the precise route for the endoscope and surgical instruments is determined, this surgery can be completed effectively with less disruption of the facet joint and musculoligamentous complex than conventional thoracic laminectomy [10].
UBE has published two articles on posterior thoracic decompression [9,10]. One article was a technical note about removal of the OLF by UBE. Kang et al. [9] described the surgical steps of UBE-assisted posterior thoracic decompression, including a discussion of the indications, benefits, complications, and ways to avoid complications. Another paper described the surgical technique and provided preliminary clinical results. Deng et al. [10] demonstrated posterior thoracic decompression by UBE in 14 patients with single-level thoracic OLF. With an average follow-up of 15.4 months, they demonstrated a statistically significant improvement in mJOA score (p<0.001) and VAS (p<0.001) with UBE. Five cases of perioperative complications were among the complications (one patient with cerebrospinal fluid leakage, two with headaches and neck pain, and two with hyperalgesia of lower limbs). According to two studies, UBE techniques are effective and safe for treating thoracic OLF.
To avoid iatrogenic spinal cord injury when performing thoracic ULBD by UBE, several principles must be followed. Following recommendations are based on the prevention of neurological deterioration. 1) When bone working is performed bilaterally, care must be taken to cover the spinal cord while preserving the LF. 2) The base of the spinous process must be removed sufficiently to ensure adequate working space, especially for contralateral decompression. 3) The placement of the surgical instrument into the stenotic spinal canal must be avoided due to the risk of cord injury. As a result, it is safe to thin the bone structure with a diamond burr. The LF and residual lamina can be removed with the double-ended elevator, and the paper-thin residual lamina can be easily removed. This technique allows for En-block resection of the LF’s deep layer without causing cord compression or dural tear. 4) Use RF probes with caution near neural structures. Surgeons must take special care when using RF probes around the spinal cord to avoid using them on neural structures with low RF power. 5) Because using Kerison Longeur to remove the OLF is dangerous, a diamond drill is used to drill through the OLF into a translucent shape. 6) If removing the OLF is difficult with dural ossification or severe adhesion, it is safe to leave the thinned portion of the OLF in place using the floating method. The dural opening must be completely sealed with a fibrin collagen patch after the OLF has been floated. 7) Fluid-related complications includes headache, neck stiffness, seizures, and cord injury [9,10]; as UBE is a fluid-mediated surgery, it is critical to monitor fluid drainage, the complications caused by fluid can be avoided by using a semi-tubular retractor. 8) Because of the technical difficulties, novice surgeons should avoid performing UBE on patients with fused or tuberous-type OLF, severe thoracic stenosis, severe OPLL. As it has clinical characteristics and a poor prognosis. 9) The thoracic cord is vulnerable due to the low canal-to-cord ratio, thoracic kyphosis that pushes the cord anteriorly, and insufficient blood supply in the watershed zone. Therefore, somatosensory and motor-evoked potentials are used to prevent cord injury.
To avoid postoperative hematoma when performing thoracic ULBD by UBE, several principles must be followed. Bone bleeding should be waxed immediately to reduce the risk of postoperative hematoma. Particular attention should be provided to epidural vessel bleeding during LF resection. Prior to LF resection, the use of the RF probe and hemostatic agents (Gelfoam or WoundClot) are sufficient to control bleeding. To prevent postoperative hematoma, the Jackson–Pratt surgical drain (100 mL) should be placed through the working portal for 1 or 2 days.
Although UBE has grown in popularity in recent years, thoracic ULBD via UBE is technically difficult and has the potential for significant outcomes. As a result, we recommend that thoracic ULBD by UBE be used only after the surgeon has sufficient experience with UBE for lumbar surgery. Due to safety and technical challenges, novice UBE surgeons should avoid challenging cases such as fused-type or tuberous-type OLF, severe thoracic stenosis or severe dural ossification. To avoid complications, the surgeon must recall anatomical landmarks, surgical techniques, and his or her level of comfort and competence with the UBE method.
CONCLUSION
In conclusion, the UBE decompression technique with unilateral approach and bilateral decompression appears to be safe and effective for treating thoracic OLF or thoracic spinal stenosis. Thoracic ULBD by UBE preserves the paraspinal muscle and ligament that would otherwise be resected during the traditional posterior approach, and it has the added benefit of reducing back pain and muscle atrophy. Although thoracic ULBD by UBE is not currently the standard treatment for thoracic OLF or thoracic spinal stenosis, this technique has the potential to be more widely used in the future.
Notes
Ethical statements
Not applicable.
Conflicts of interest
No potential conflict of interest relevant to this article.