A Clinical Pilot Study Showing the Safety and Efficacy of Intramuscular Injection of Atelocollagen for Prevention of Paraspinal Muscle Atrophy after Spine Surgery
Article information
Abstract
Objective
The objective of this study was to assess the safety and efficacy of intramuscular injection of atelocollagen for the prevention of paraspinal muscle atrophy after spine surgery. Atelcollagen has been widely used as an intradermal filler to restore soft tissue defect. Many studies demonstrated that atelocollagen provides good therapeutic results by promoting cell proliferation and enhances the healing effect on injured connective tissues such as tendons and fasciae, while causing few complications.
Methods
A total of 118 patients who underwent single level of posterior lumbar interbody fusion (PILF) between December 2017 and April 2019 were retrospectively reviewed. In the study group of 60 patients, 3 mL of gel-type 3% atelocollagen solution was prepared and injected into the multifidus muscle during wound closure. Clinical efficacy was evaluated by the improvement of back pain, elevation of a muscle enzyme, and inflammatory markers. Radiologic efficacy was evaluated with a comparison of density and cross-sectional area (CSA) of multifidus and erector spinae muscle in CT images.
Results
Visual analogue scale (VAS) scores for back pain was not significantly lower in the study group postoperatively compared with the control group. The reduction of postoperative paraspinal muscle density and CSA was significantly lower in the study group. The serum level of muscle enzyme and inflammatory markers were significantly lower in the study group. No major procedure-related complications were observed during the follow-up period.
Conclusion
Intramuscular injection of atelocollagen is safe and feasible for the prevention of paraspinal muscle atrophy after spine surgery. This novel method seems advantageous for accelerating wound healing without causing inflammation.
INTRODUCTION
Recently spine surgeons have been concerned about the surgical approach-related morbidity resulting from an iatrogenic paraspinal muscle injury in posterior lumbar surgery. Many studies have explained the mechanisms of the injury and reported new techniques to prevent the injury [1-3], but there is still not an effective and established treatment proven yet.
Collagen is a triple helix polymer protein that makes up 30 percent of the body’s total protein [4]. Collagen is a structural and biological component of tissues including cartilage, bone, vessels, skin, and tendon. Collagen fibers act to transmit forces, dissipate energy, and prevent mechanical failure in connective tissues [4,5]. A collagen molecule has an amino acid sequence called a telopeptide at both N- and C-terminals, which confers most of the collagen’s antigenicity. If this collagen with telopeptide is injected into the human body, an immune response can occur because of the antigenicity of the telopeptide at N- and C-terminals [6].
Atelocollagen is a material that is extracted from animal skin and is prepared by protease or pepsin treatment to remove this antigenic telopeptide region from both ends of the collagen molecule. Highly purified atelocollagen is low in immunogenicity because it is free from telopeptides and has many advantages for biocompatibility and optimizing collagen-cell interaction for efficacy and lower side effects [7]. Studies using human subjects and experimental animals have shown that atelocollagen provides good therapeutic results by promoting cell proliferation and early epithelialization while causing little rejection and few complications [8].
Over the past decade many reseachers have conducted studies in tissue engineering using collagen to enhance muscle recovery. However, in our knowledge, there is not a single study on prevention of paraspinal muscle injury after spine surgery. Our primary hypothesis was that atelocollagen may affect postoperative muscle recovery by promoting local stem cell and myoblast proliferation without adverse events. The object of this study is to assess the safety and efficacy of intramuscular injection of atelocollagen for the prevention of paraspinal muscle atrophy after spine surgery.
MATERIALS AND METHODS
Approval of the institutional review board for this study was obtained (CMC IRB No. PC22RISI0010). Patients who underwent single level mini-open posterior lumbar interbody fusion (PILF) from December 2017 and April 2019 at Seoul St. Mary’s Hospital were identified and retrospectively reviewed. Inclusion criteria for the surgery were degenerative indications requiring a fusion procedure such as segmental instability, spondylolisthesis, and disc degeneration disease with herniation and/or spinal stenosis. Patients with previous spine surgery, spine trauma, infection, ankylosing spondylitis, malignancy, and congenital spinal deformities were excluded from the study. To eradicate the bias about paraspinal muscle quality, patients with diagnosis of sarcopenia were also excluded. The diagnosis of sarcopenia was done using diagnostic criteria proposed by The Asian Working Group for Sarcopenia (AWGS) [9]. All patients were observed clinically and radiologically for a minimum of 12 months. Informed consent from the study pateints were deemed exempt from requirment due to restrospective study design.
All operations were conducted by a single neurosurgeon (J.W.H.) with the same surgical protocol (Figure 1). All patients underwent mini-open PLIF in the prone position on a Jackson table. Following a midline skin incision and bilateral paraspinal muscle dissection, decompressive surgery consisting of total laminectomy, medial facetectomy, and foraminotomy bilaterally was done. The thecal sac was retracted gently to expose a corridor to the disc space. The endplates and disc space were then prepared followed by the insertion of PEEK cages (OIC; Stryker, Portage, MI, USA) filled with autograft bone and demineralized bone matrix (DBM; AllomatrixTM DR; Wright, Memphis, TN, USA) bilaterally. Additionally, percutaneous pedicle screws with a vertical axis and detachable extender (AnyPlus® MIS percutaneous pedicle screw system; GS Medical, Cheongju, Korea) were inserted under C-arm guidance.

Illustrative case of mini-open PLIF of 46 years old female patients in the study group. Posteoprative MRI demonstrated absence of obvious paraspinal muslce atorphy at the index level.
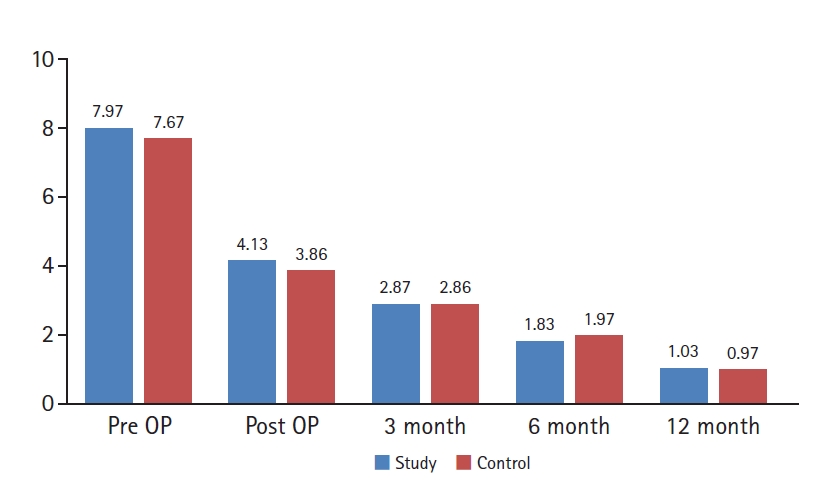
For the study group, 3 mL of 3% atelocollagen (Coltrix® Tendoregen; Ubiosis, Seongnam, Korea) was injected at the paraspinal muscles. After the main operation procedures and layer by layer muscle closure, atelocollagen from a pre-filled syringe was injected at the paraspinal muscle using an 18 gauge needle, 1 cm from midline facial closure site bilaterally along the operation scar. Each injection points were at least 1 cm apart longitudinally and mean dosage of 0.2 mL was injected at each point (Figure 2). The subcutaneous layer and skin closure were followed accordingly after the injection. On the other hand, the same procedures were performed except atelocollagen injection after the muscle closure, in the control group. Bilateral submuscular drainage was inserted in all patients. Postoperatively, all patients were applied with routine 1-day intravenous antibiotics and were admitted for 2 weeks until wound stich out. The routine rigid back brace (usually lumbar-sacral orthosis) were applied to all patients for 3 months and typical postoperative managements such as medications; non-steroidal anti-inflammatory drugs, muscle relaxants etc. and physical therapy were administered.
Injection of atelocollagen from a pre-filled syringe using an 18 gauge needle, 1 cm from midline bilaterally along the operation scar.
Clinical efficacy was assessed by an independent third party, an experienced clinical study coordinator, who was blinded to all relevant knowledge of the patients, using a visual analog scale (VAS) for back pain.
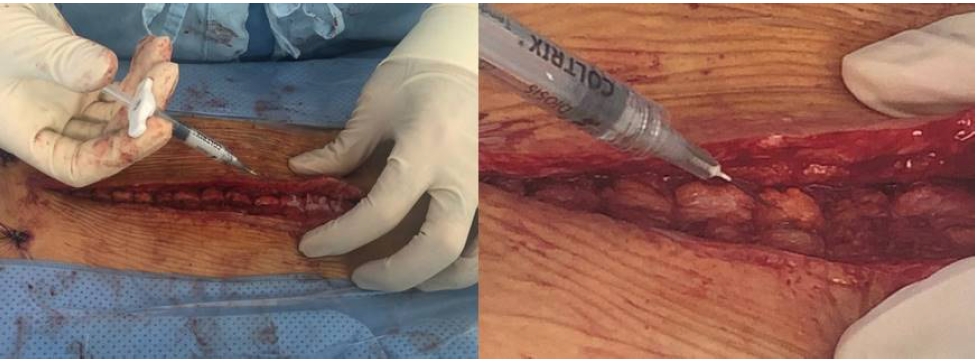
Radiologic efficacy was evaluated with computer tomography (CT) with 3D reconstruction comparing images taken preoperative and 12 month postoperatively. The cross-sectional area (CSA) of multifidus and erector spinae muscles was measured in the mid-intervertebral index disc level at the axial CT image. The regions of interest (ROI) of individual muscles were measured by placing polygon points around the outer margins of the muscles to avoid metallic artifacts (Figure 3). The density of the multifidus and erector spinae muscles was measured in Hounsfield units (HU) on both sides at mid-intervertebral index disc level and the mean value was obtained. It was evaluated by measuring the mean density in the ROI, using a 6-mm circle in the center of the muscle mass without visible fat deposits (Figure 4). The radiologic parameters were measured from L2-3 to L5-S1 level in each patient and the mean value was obtained for the statistical analysis. All radiologic measurement was done by observing the images obtained on a digital radiographic image displayed on a Picture Archives and Communication System (PACS terminal; nU PACS V. 1.0.0.36.17, 2019; Taeyoungsoft Inc., Anyang, Korea) and performed twice by two independent observers.
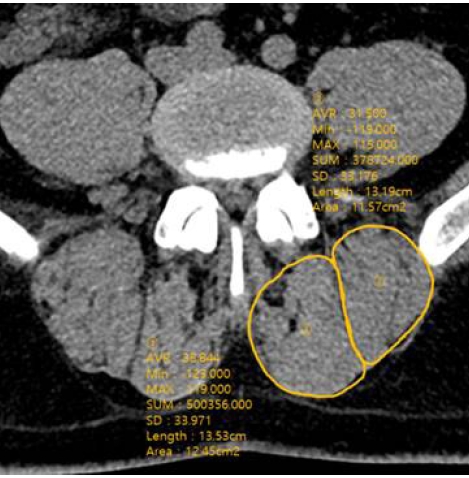
The cross-sectional area (CSA) of multifidus and erector spinae muscles was measured in the mid-intervertebral index disc level at the axial CT image.
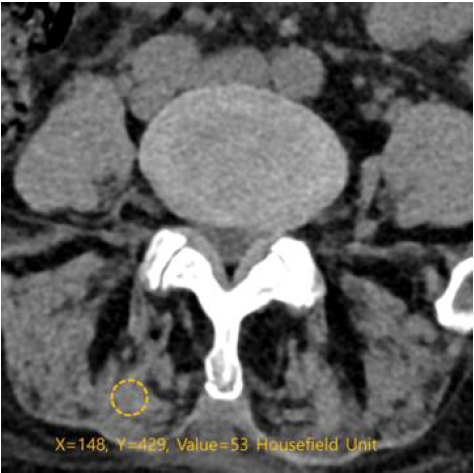
The density of the multifidus and erector spinae muscles was measured in Hounsfield units on both sides at the index level. It was evaluated by measuring the mean density in the region of interest, using a 6-mm circle in the center of the muscle mass without visible fat deposits.
Laboratory examination including standard muscle enzymes (creatine kinase [CK] and lactate dehydrogenase [LDH]) and additional serum inflammatory markers (C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]) were performed in all patients. The safety was assessed with a survey and medical document review on all adverse events over 12 month.
The data were described as mean±SD. All statistical comparisons were 2-tailed, and the threshold for statistical significance was set at p<0.05. The inter-observer reliability was examined using 1-way analysis of variance, and the intra-class correlation coefficient (ICC). Regression logistic analysis was used to evaluate the correlation of CSA and muscle density with the patient's age, gender, BMI, smoking status and comorbidities.
RESULTS
A total of 118 patients received single level mini-open PLIF (60 men and 58 women) were retrospectively reviewed. The mean age at the time of the surgery were 65.4 years in the study group and 64.2 years in the control group. The most common operation level was L4-5 in the both groups. Patient demographics and perioperative data were described in Table 1.
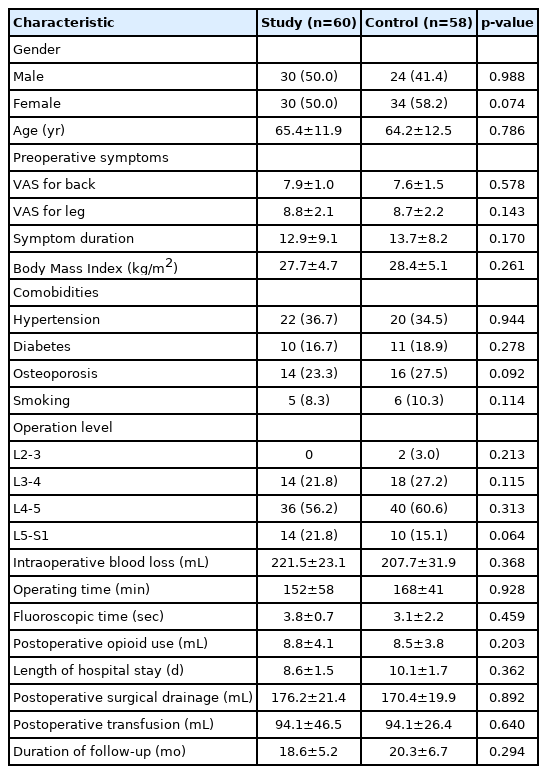
Posteopratively, both group demonstrated statistically singnificant improvement of clinical outcome in mean 12 month follow-up. There was no significant difference in the VAS scores between the two groups in all time point (Figure 5).
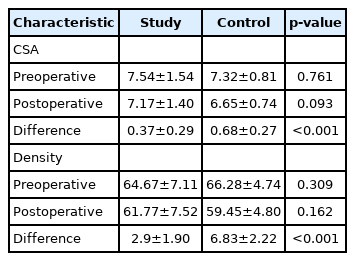
There was no statistically significant difference in the mean CSA of paraspinal muscles between the study group and the control group and both group demonstrated singnificant decrease postoperatively. However, the reduction of postoperative paraspinal muscle density was significantly lower in the study group (p<0.001; Table 2). Moreover, there was no statistically significant difference in the mean density of paraspinal muscles between the study group and the control group and both group demonstrated singnificant decrease postoperatively. The difference in pre- and postoperative density of the paraspinal muscles in the study group was significantly higher compared in the study group (p<0.001; Table 2).
Mean cross sectional area (CSA) and density of paraspinal muscles measured with computer tomography (CT) with 3D reconstruction
The assessment of inter-observer reliability showed good agreement for the muscle volume (ICC=0.79), and excellent agreement for the muscle density measurement (ICC=0.92), indicating the measurements were reliable. In regression analysis, correlation between CSA and muscle density with demographic variables were not significantly associated.
The serum level of muscle enzyme and inflammatory markers demonstrated significant increment postoperatively in both groups, which gradually decresed until final follow-up. The amount of reduction in CK were significantly higher in the study group at 3 month, 6 month and 12 month, 6 month and 12 month in LDH. However, the differences of pre- and postoperative inflammatory markers between the two groups were not statistically significant during the entire follow-up period (Figure 6).
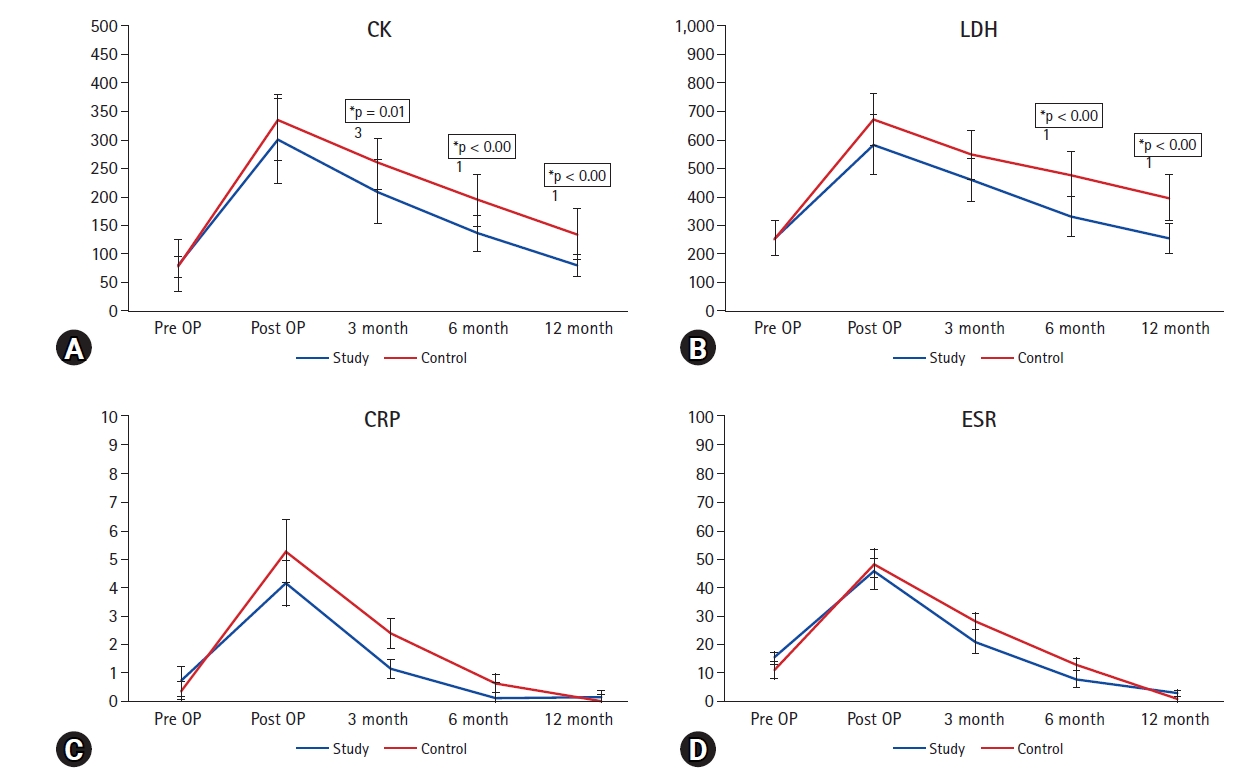
The serum level of muscle enzyme and inflammatory markers demonstrated significant increment postoperatively in both groups, which gradually decresed until final follow-up. The amount of reduction in muscle enzyme were significantly higher in the study. However, the differences of pre- and postoperative inflammatory markers between the two groups were not statistically significant during the entire follow-up period. CK: creatine kinase, LDH: lactate dehydrogenase, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, OP: operation.
No procedure-related complications were observed during the entire follow-up period except two cases of subclinical infection treated with antibiotics without sequele in the study group.
DISCUSSION
Paraspinal muscle atrophy after lumbar spine surgery is an well-known cause of posteoprative axial back pain and adjacent segment degeneration [10-12]. The lumbar paraspinal muscle is important in maintaining lumbar segmental stability, and its defect and increased intramuscular fat infiltration are believed to cause disc degeneration [11,13]. Recently spine surgeons have tried to reduce iatrogenic paraspinal muscle atrophy by minimizing paraspinal muscle dissection using various methods [1,2]. Although, these minimally invasive surgical techniques seemed to be effective in maintaining the volume of paraspinal muscles after surgery to some degree, one cannot always use these techniques in numerous situations.
Muscle regeneration occurs in interrelated and time-dependent phases; degeneration, inflammation, regeneration, remodeling, and maturation. After initial degeneration phase, necrotic cell death stimulates a local inflammatory response [14,15]. The inflammatory response of injured skeletal muscle plays an important and critical role in muscle homeostasis and regeneration and involves the recruitment of specific myeloblastic cells within the injury site [16]. These inflammatory responses occur during 24 hours to 2 days after initial injury, followed by regeneration, remodeling and maturation phase. The dominant role in muscle regeneration is played by the muscle stem cells known as satellite cells, which reside between the basal lamina and sarcolemma of myofibers [15,17]. Satellite cells are activated in response to both physiological stimuli and pathological conditions to recruit myoblasts that can either fuse with existing myofibers repairing damaged muscle fibers, or alternatively fuse to each other to form new myofibers [18,19].
Theoretically local injection of Atelocollagen proliferate recruitment of myoblasts and satellite cell and act as scaffold for paraspinal muscles and fascial regeneration during inflammatory and regeneration phases [20-22]. Recent in vivo studies indicate that atelocollagen scaffolds provides a suitable substrate for mesenchymal stem cell attachment and enhancing chondrogenic differentiation [23,24]. However, the precise mechanism of healing effect after atelocollagen injection is yet to be clarified.
Several studies have demonstrated surgical role of atelocollagen and promising clinical results have been reported [8,16,21,23,25]. In a recent study using atelocollagen in orthopaedic surgery, Suh et al. [26] suggested repair after using patch-type atelocollagen between the torn rotator cuff and bone using rabbits. As a result, the group that used atelocollagen showed pathological and biomechanical superiority. However, uptodate there is not a single study about effect of atelocollagen injection on parapsinal muscle after spine surgery.
Our study demonstrated that injection of atelocollagen led to significant improvement in postoperative paraspinal muscles volume and density. These results suggest that atelocollagen injection after spinal surgery may be a viable option to reduce postoperative parapsinal muscle atrophy and enhance tissue healing. However, these radiologic findings did not affect clinical outcomes. The possible reasons are mainly because our study sample size is too small and mini-open PLIF is relatively less muscle invasive surgical technique using percutaneous pedicle screw fixation. Although there was no significant improvement of VAS score after injection of atelocollagen, this study proved initial safety of type 1 atelocollagen injection to paraspinal muscles as a clinical pilot study. Besides, the study group did not show inferiority compared with the control group regarding clinical and radiologic outcomes.
Our study has several strengths. This study is the first clinial trial examining the effect of atelocollagen injection on spine surgery. To our knowledge, this the first clinical pilot study to evaluate effect of atelocollagen injection on parapsinal muslce damage. Moreover, we used CT scan to precisely assess postoperative paraspinal muscle volume and density. Most of all, compared to other minimally invasive surgical techniques, the atelocollagen injetion is less time-consuming, more cost-effective, and much easier method.
There are sevral limitations to this study. First, this study is a retrospective study and the sample size is maybe too small and follow-up period of 12 months maybe too short to assess the precise benefits of using atelocollagen. In the future, a prospective study with a larger sample size and longer follow-up period will be needed to validate the result of this study. Second, we could not find out the direct relevance between clinical improvement in VAS score and the volume of paraspinal muscle. Although, the obtained results were not statistically sginificant, further studies on the use of atelocollagen will be needed in the future.
CONCLUSION
Intramuscular injection of atelocollagen is safe and feasible method to prevent paraspinal muscle atrophy after spine surgery. This novel method seems advantageous for accelerating wound healing without causing harzadous inflammation. Prospective controlled trial with larger data samples are warranted in the future.
Notes
Ethical statements
This study has been approved by the institutional review board of CMC, Catholic university of Korea (CMC IRB No. PC22RISI0010).
Conflicts of interest
No potential conflict of interest relevant to this article.