Full-endoscopic Trans-Kambin’s Triangle Lumbar Interbody Fusion: Technique and Review of Literature
Article information
Abstract
Transforaminal full-endoscopic(fullendo) lumbar surgery was first introduced as a method for performing discectomy. More recently, it has been used for decompression of lumbar spinal canal stenosis. The fullendo technique can also be performed for lumbar interbody fusion (LIF) through Kambin’s triangle. Similar techniques have been used to insert a cage using fullendo surgery and are described variously in the literature as percutaneous endoscopic LIF, percutaneous endoscopic transforaminal LIF, full-endoscopic LIF, and full-endoscopic transforaminal LIF. Given that a cage is inserted through Kambin’s triangle, we have proposed that this method be known as fullendo trans-Kambin’s triangle lumbar interbody fusion (fullendoKLIF). We have recently created a fullendo-KLIF surgical system. In this paper, we describe our surgical procedure, report the initial clinical results, and review similar fullendo LIF techniques reported in the literature.
INTRODUCTION
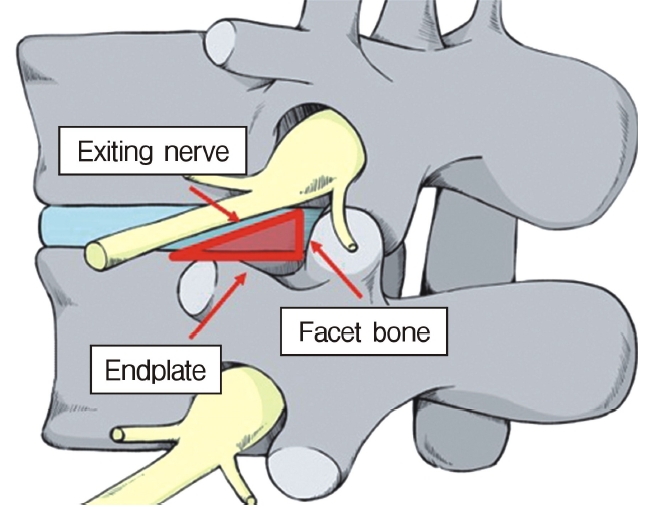
In the 1980s, Kambin identified the anatomic corridor now known as Kambin’s triangle [1,2] as a minimally invasive method for accessing the intradiscal space(Figure 1). This route is different from the traditional interlaminar approach and was introduced after percutaneous discectomy [3], which was first described by Hijikata [1,2]. After several trials that used an endoscopic approach to negotiate Kambin’s triangle, the currently used single-portal full-endoscopic surgical system was developed by Yeung [4,5].
Transforaminal (TF) full-endoscopic (fullendo) surgery was initially used for discectomy [4-6]. The indication for TF-fullendo was then expanded to include decompression of spinal canal stenosis [7]. Foraminal stenosis was the initial target for decompression using TF-fullendo surgery because the intervertebral foramen is anatomically close to the entry point for the TF approach [8,9]. The next indication was lateral recess stenosis [7]. To decompress the lateral recess using the TF approach, the ventral side of the facet joint needs to be removed [10,11]. Therefore, Sairyo et al. coined the term “ventral facetectomy” to describe TF-fullendo surgery [10]. The final step was decompression of central canal stenosis. The method developed by Sairyo et al. [12] included removal of all of the superior articular process and partial removal of the inferior articular process using a TF approach, allowing wide exposure of the thickened ligamentum flavum compressing the cauda equina. The thickened ligamentum flavum can be removed using a specially designed Kerrison rongeur. This surgical technique is known as “TF-fullendo lumbar undercutting laminectomy”. Nowadays, all types of spinal canal stenosis can be treated by fullendo surgery using a TF approach.
TF-fullendo surgery can also be performed to treat intradiscal disorders. The first such application of TF-fullendo surgery was when Ito et al. [13] successfully treated 15 cases of pyogenic discitis using full-endoscopic debridement and irrigation. The next application was intradiscal therapy in patients with severe annular tears and discogenic pain [14,15]. The site of the annular tear was electrically coagulated using a bipolar radio-pulse device, a technique that has become known as “TF-fullendo thermal annuloplasty”. Most recently, TF-fullendo has been used for disc cleaning in patients with type 1 Modic change [16]. After TF-fullendo cleaning surgery, low back pain disappears and inflammation seen on short tau inversion recovery images resolves. Nowadays, TF-fullendo surgery is performed worldwide for pyogenic discitis, discogenic pain, and type 1 Modic change [13-16].
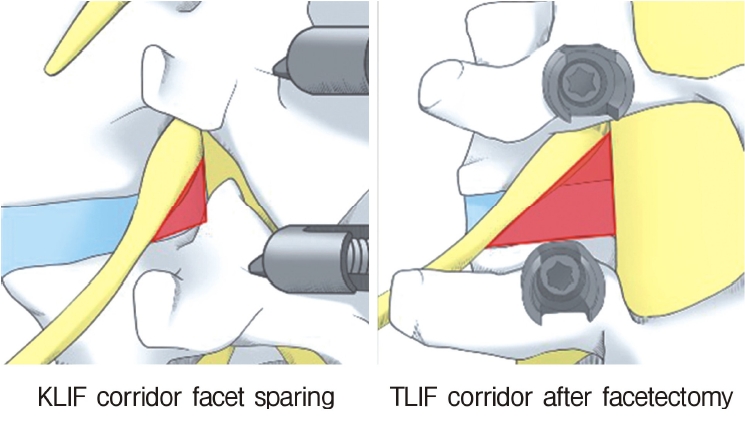
The final challenge was insertion of a cage through Kambin’s triangle. Various full-endoscopic lumbar interbody fusion techniques has been reported. For example, “percutaneous endoscopic lumbar interbody fusion” (PELIF) was described by Nakamura et al. in 2017 [17] and subsequently by Wu et al. in 2018 [18] while Youn et al. [19] used the term “full-endoscopic lumbar interbody fusion” (FELIF), also in 2018. In the same year, Nagahama et al. [20] described their method, which included introducing an ovary-shaped cannula, and named it “percutaneous endoscopic transforaminal lumbar interbody fusion” (PETLIF); Kamson et al. [21] subsequently referred to this technique as “full-endoscopic transforaminal lumbar interbody fusion” (FETLIF). When describing a similar technique, Lewandrowski et al. [22] proposed the term “lordotic endoscopic wedge lumbar interbody fusion” (LEWLIF). Despite their different names, all these LIF techniques involve insertion of a cage through Kambin’s triangle under full-endoscopic guidance (Figure 2). Therefore, the term “trans-Kambin triangle LIF” (KLIF) was finally proposed in 2019 [23,24].
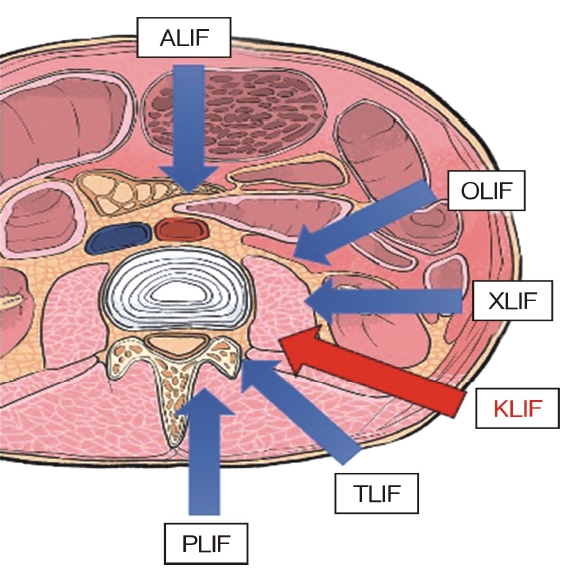
Anatomic site where the cage is inserted during a full-endoscopic trans-Kambin’s triangle lumbar interbody fusion procedure.
We have developed a Fullendo-KILF system (Surgical Spine Inc., Tokyo, Japan) that can be used to insert any type of cubic or expandable cage safely while protecting the exiting nerve root. In this paper, we introduce our fullendo-KLIF procedure and report our early clinical results for this system.
SURGICAL PROCEDURE
1. Indications
The best candidates for fullendo-KLIF at present are patients with a single-level disorder. Single-level fusion for conditions such as spondylolisthesis, scoliosis, discogenic pain, and Modic change can now be performed using this method.
Decompression is achieved indirectly by posterior reduction using a percutaneous pedicle screw system and inserting a cage. If direct decompression is needed for the exiting and/or traversing nerve root, TF-fullendo ventral facetectomy and/or foraminotomy is performed, respectively.
2. Technique
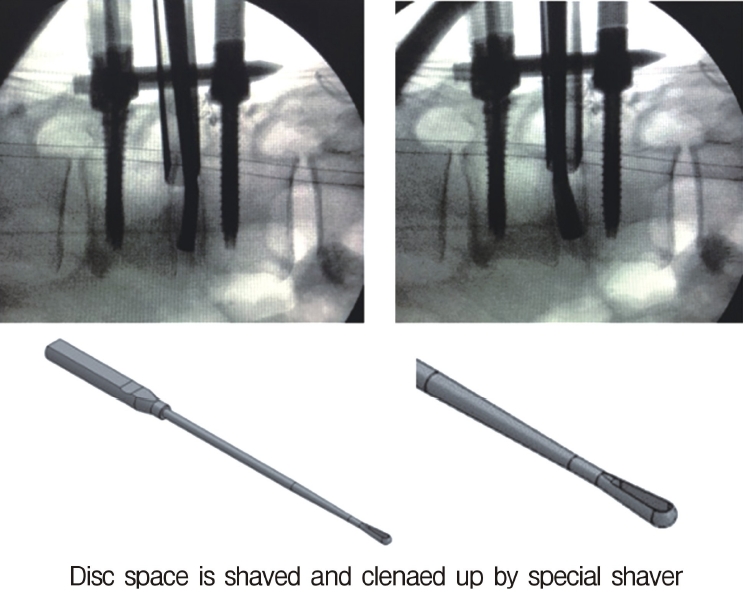
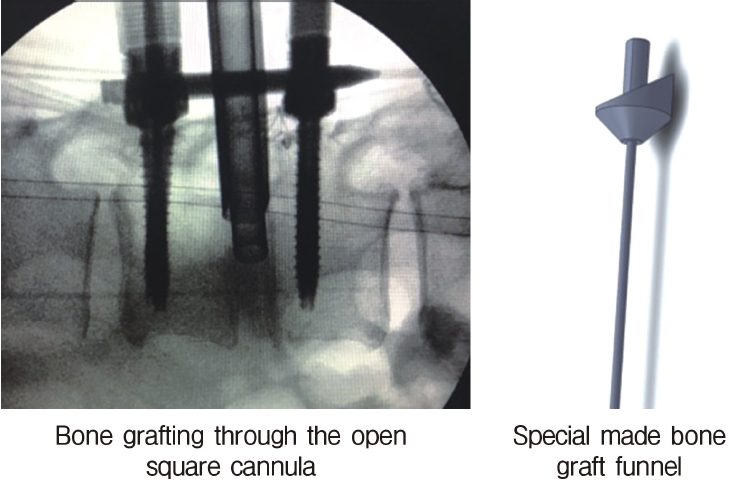
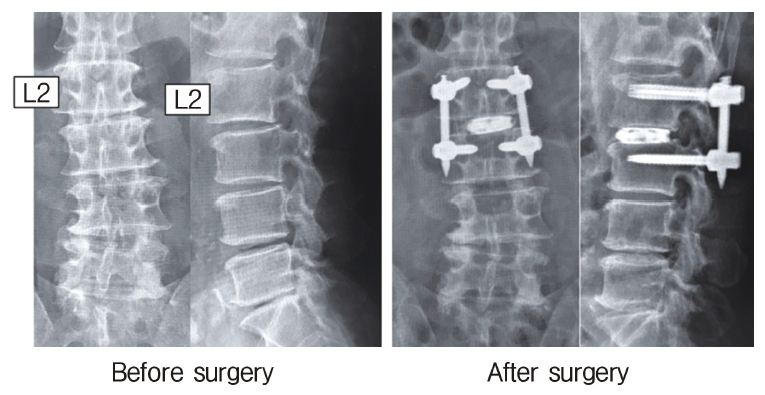
Neuromonitoring is performed throughout the surgery to avoid damage to the exiting nerve root. The procedure is explained here using a case of L2 degenerative spondylolisthesis as an example. Before fullendo-KLIF surgery, four percutaneous pedicle screws are inserted at L2 and L3 (Figure 3). The slippage is reduced and the disc height is extended as far as possible. The cannula of the full-endoscope is then docked on the superior articular process as shown in Figure 4. After adequate foraminoplasty, a guidewire with a safety ball at the tip is inserted into the disc(Figure 4, right panel). Adequate foraminotomy is then confirmed endoscopically. As shown in Figure 5, we usually create 12 mm of disc distance(4 times the 3-mm diameter of the surgical drill). An 8- to 10-mm cannulated spacer is inserted through an S-guide to enlarge the disc space (Figure 6). Using this device, the disc space is further widened to 10 mm. Using the spacer, an open square cannula is inserted just inside the disc (Figure 7). As shown in the figure, the open cannula completely protects the exiting nerve root. A specially designed curette or disc shaver (Figure 8) is then passed through the open cannula to empty the disc space and to curette the disc endplate. An autogenic or allogenic bone graft is implanted using a specially designed bone graft funnel (Figure 9, left panel). An expandable cubic cage is then inserted through the open cannula (Figure 10, left panel) and expands inside the disc (Figure 10, right panel). The cage has a width of 10 mm and a length of around 30 mm. Plain radiographs obtained before and after surgery are used to confirm that the slipped vertebra is reduced and the disc space is opened up (Figure 11).
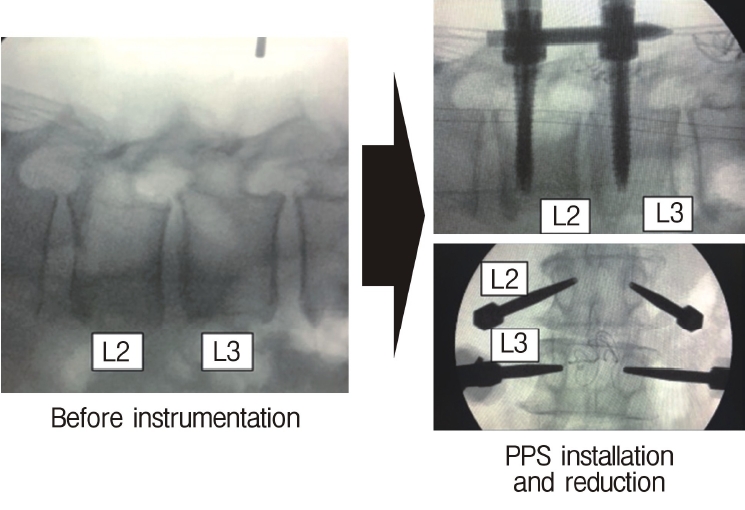
C-arm image obtained during surgery. Forward slippage of the vertebral body at L2 (left panel) is reduced after insertion of percutaneous pedicle screws (right panel).
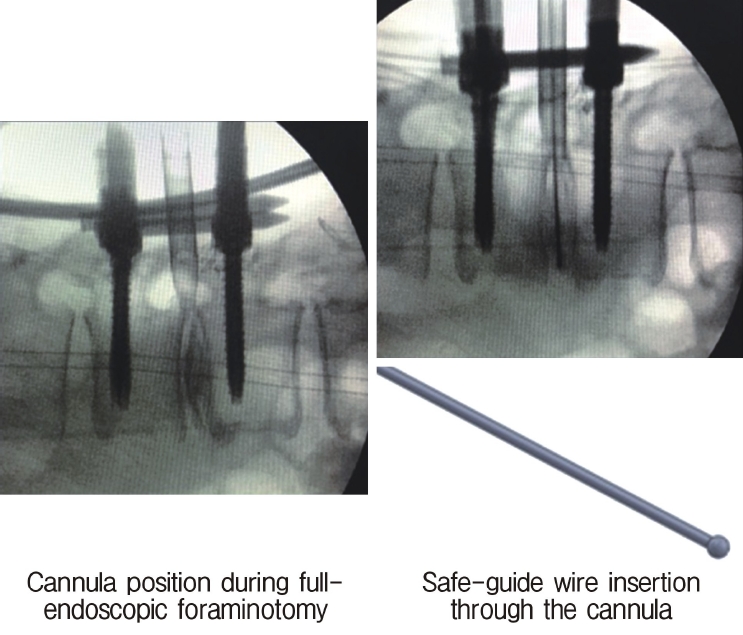
C-arm image obtained during surgery. The left panel indicates the position of the cannula and the right panel shows insertion of a safety guidewire through the cannula.
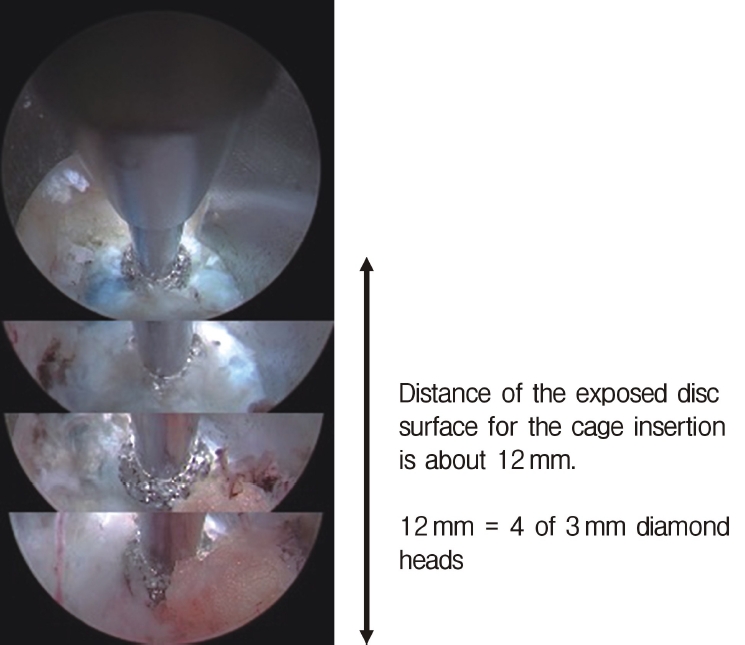
Endoscopic view after foraminotomy. Usually, a disc distance of 12 mm (4 times the 3-mm surgical drill diameter) is created for safe insertion of the cage.
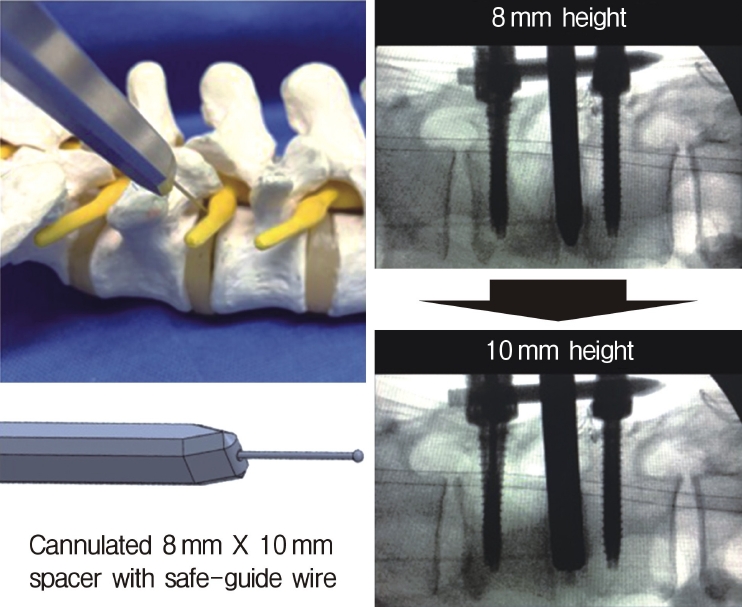
Insertion of the cannulated spacer. Through the S-guide, an 8- to 10-mm cannulated spacer is inserted to widen the disc space to 10 mm.
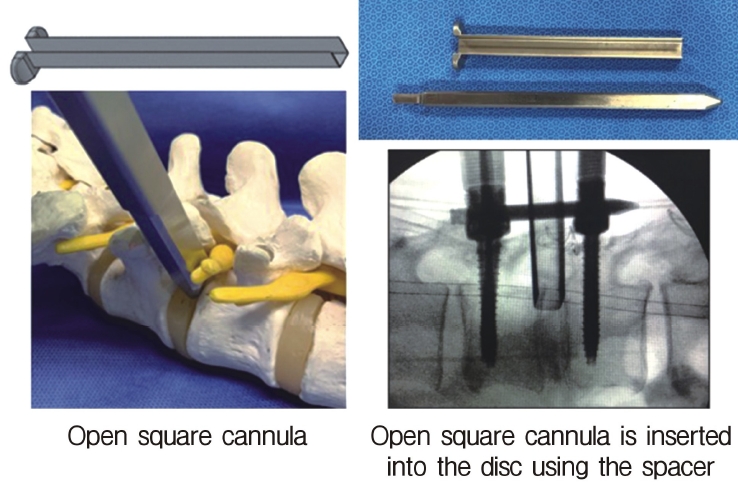
Insertion of the open square cannula just inside the disc using the spacer. The cannula protects the exiting nerve root.
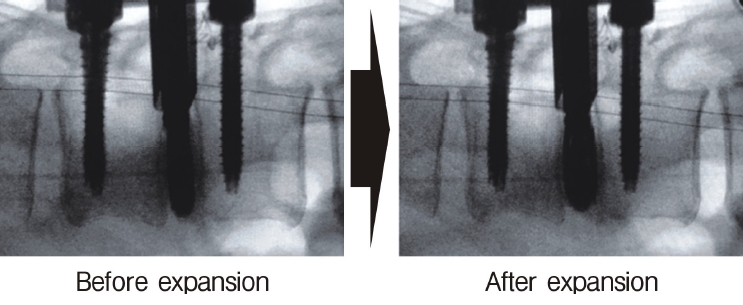
Insertion of the cage through the open square cannula (left panel), which expands inside the disc (right panel).
METHODS
Between 2018 and May 2020, we performed fullendo-KLIF surgery in 25 patients. The PETLIF system designed by Nagahama et al. [20] was used to insert the cage in the first 13 patients (6 male, 7 female). Since May 2020, we have used the fullendo-KLIF system in 12 patients (5 male, 7 female). The surgical complications and clinical outcomes in these patients were evaluated.
RESULTS
In our first patient, fullendo-KLIF surgery was performed at L4/5 using the PETLIF system. At the time of surgery, the patient complained of transient exiting nerve irritation at L4, which improved within 2 weeks. There were no other complications, such as dural tear, traversing nerve injury, infection, or hematoma, in subsequent patients. Therefore, the overall complication rate was 4.0% (1/25) for both types of KLIF (i.e., 7.7% [1/13] for the PETLIF system and 0% for the fullendo-KLIF system). In retrospect, the complication that occurred in the first case probably reflects the surgical learning curve.
We have already reported the clinical results of the initial 10 cases who underwent fullendo-KLIF surgery using the PETLIF system [24]. The visual analog scale (VAS) scores for low back pain and leg pain significantly (p<0.05) decreased from 6.9 to 0.9 and from 6.0 to 0.9, respectively. The clinical outcome was excellent in 8 cases and good in 2 according to the modified MacNab criteria. The clinical outcome was good or excellent in all 12 patients treated with the fullendo-KLIF system, although the follow-up duration was less than 1 year.
DISCUSSION
1. Development of Transforaminal Full-endoscopic Spine Surgery
TF-fullendo surgery was first used for discectomy [4-6]. Initially, a herniated nucleus pulposus (HNP) at the disc level without migration was considered a good indication for inside-out TF-fullendo discectomy [6]. Later, when the foraminoplasty technique was introduced [25], a migrated HNP was also accepted as an indication for TF-fullendo discectomy [26]. Choi et al. performed TF-fullendo surgery in 59 patients with migrated HNP, which they corrected using a foraminoplasty technique, and found that 91.4% of patients had a satisfactory outcome. At first, HNP at L5-S1 was considered too difficult to treat because of obstruction by the iliac crest [27]. However, it was found that HNP at L5-S1 could be removed using foraminoplasty [28].
Next, the surgical indications were expanded to include decompression of spinal canal stenosis. Foraminal stenosis was the initial target for anatomical reasons. As far as we are aware, the first report on the foraminoplasty technique was published in 2003 by Ahn et al. [29], who performed posterolateral endoscopic foraminal decompression using a bone reamer, endoscopic forceps, and a laser under fluoroscopic guidance. However, other surgeons did not adopt this technique. With the advent of the surgical drill, further advances in bony decompression surgery were made. Lewandrowski et al. [30] subsequently used the surgical drill for foraminal decompression, as did Yamashita et al. [31].
Lateral recess stenosis was the next target. The ventral side of the facet joint needs to be removed to decompress the lateral recess using the TF approach [10,11]. Sairyo and colleagues [10,11] then used the high-speed surgical drill to remove the hypertrophied facet joint, which introduced the concept of ventral facetectomy. Xie et al. [32] developed an alternative strategy for decompression of the lateral recess via the TF approach, which entailed using a 10-mm trephine rather than a high-speed drill. Finally the indications for TF-fullendo surgery were expanded to include central canal stenosis. Sairyo et al. [12] reported a procedure they described as TF-fullendo lumbar undercutting laminectomy for surgical decompression of the central stenosis. Therefore, any type of spinal canal stenosis is now a good indication for fullendo surgery.
Surgical intradiscal therapy started with debridement of infection and expanded to include thermal annuloplasty, disc cleaning surgery, and finally insertion of a cage into the dissected disc space.
2. Nomenclature for Full-endoscopic Trans-Kambin's Triangle Lumbar Interbody Fusion
Several LIF techniques can be used to insert a cage in the intradiscal space and are named according to the anatomic site at which the cage is inserted. Examples include posterior LIF, anterior LIF, transforaminal LIF, direct lateral LIF, extreme lateral LIF, and oblique lateral LIF. Full-endoscopic insertion of a cage into the disc space via Kambin’s triangle started in the late 2010s. This facet-sparing technique has been described variously as PELIF, PETLIF, FELIF, FETLIF, or LEWLIF, and is now known as KLIF [23,24].
The anatomically close relationship between the trans-Kambin technique and the TF technique has led to some confusion between KLIF and TLIF. Both methods include insertion of a cage through Kambin’s triangle; however, Nagahama et al. [20] used the term “percutaneous endoscopic TLIF” while Kamson et al. [21] used the “full-endoscopic TLIF”. Moreover, Morgenstern et al. [33] described their facet-sparing technique for inserting a cage through Kambin’s triangle as “percutaneous TLIF” [33]. Tumialan et al. [34] pointed out that although the trans-Kambin and TF approaches seem to be similar, there are marked differences in the angle of trajectory and the bone work required. The TLIF approach entails widening Kambin’s triangle and cannot be performed without total facetectomy. Therefore, our procedure is called KLIF rather than TLIF (Figure 12).
On the other hand, there has been reported the real full-endoscopic TLIF [35,36]. According to the report by Wu et al. [35] they do total facetectomy under the guidance of endoscope; then, a cage is inserted into the disc space. The authors called the technique as endo-TLIF. They clearly differentiate the technique from the trans-Kambin Endo-LIF. Our fullendo-KLIF is called transKambin endo-LIF in the paper. The other report is by Kim et al. [36]. Similarly they do total facetectomy with endoscope and insert a cage into the disc. They call the technique is endo-TLIF.
Actually, during their surgical procedure by We et al. [35] and Kim et al. [36], total facetectomy is conducted prior to the cage insertion. Thus, this should be the endo-TLIF, not KLIF.
3. Clinical Efficacy of Fullendo-KLIF
In our initial 10 cases, the respective VAS scores for low back pain and leg pain decreased from 6.9 to 0.9 and from 6.0 to 0.9. In these patents, the clinical outcome was excellent in 10 cases and good in 2 according to the modified MacNab criteria. The clinical outcome was also acceptable when the fullendo-KLIF system was used, albeit during a short follow-up period. Nakamura et al. [35] performed PELIF (trans-Kambin LIF) in 21 patients (8 male, 13 female), who were followed up for a mean of 20.3 months. The mean VAS scores improved from 6.1 to 1.9 for lower back pain in patients with severe disc degeneration without spondylolisthesis and from 7.6 to 1.0 for lower extremity symptoms in patients with spondylolisthesis. The clinical outcomes after these types of surgery are similarly good [17-22].
Of great concern is exiting nerve root injury (ENRI) during KLIF surgery. Lewandrowski et al. [22] reported the highest rate of ENRI during cage insertion via Kambin’s triangle (60.4%; 29/48 cases) and Morgenstern et al. [33] reported the second highest rate (22%). Avoiding ENRI would be the most important determinant of the safety of KLIF surgery. As shown in Figure 5, the disc surface area can be observed endoscopically during the foraminotomy procedure. The width of the disc space from the facet bone to the exiting nerve must be at least 10 mm, because width of a cage is a 10 mm. Therefore, for safe insertion, we create a disc surface with a width of 12 mm. ENRI has not occurred in the 12 patients we have treated using the fullendo-KLIF system.
CONCLUSION
In this paper, we have introduced our technique for inserting a cage into the disc space via Kambin’s triangle under full-endoscopic guidance. We have named this procedure fullendo-KLIF (full-endoscopic trans-Kambin triangle interbody fusion). This is the most minimally invasive LIF surgery presently available and has acceptable clinical results while causing minimal damage to the back muscles. Clinical results were satisfactory. Endoscopic guidance is expected to enable safer cage insertion.
Notes
No potential conflict of interest relevant to this article.