Overview of Tips in Overcoming Learning Curve in Uniportal and Biportal Endoscopic Spine Surgery
Article information
Abstract
There is increasing trend of spine surgeries performed with uniportal and biportal endoscopic spine surgery. Despite its good outcomes and supportive literature, the proportion of spine surgeons regularly doing endoscopic spine surgeries is relatively low. The main limitation is the steep learning curve in the early phase of endoscopic spine surgeries practice. There is paucity of literature in discussion of tips in overcoming these 2 schools of endoscopic spine surgery practice. In this tips and tricks overview, the authors highlights the key differences these 2 main school of endoscopic spine surgeries practice with specific discussions pertaining to “BIPORTALS”: the areas of basic medium of surgery, instruments for visualization and working, placement of portal(s), outflow of fluids, route of approach, technical challenges, anatomical challenges, location of lens and light source and start point to work. A ladder of progression of endoscopic spine surgery is proposed to effectively ease into the practice of endoscopic spine surgery.
INTRODUCTION
There is a growing trend and demand for minimally invasive spine surgery and in particular endoscopic spine surgery [29]. The common goal of achieving maximal amount of benefit with the least amount of soft tissue damages is the main aim of endoscopic spine surgery [30,54]. The advantages of endoscopic spine surgery is provided by water irrigation surgery, improvements in visualization with magnification of spinal structures, safe delivery of energy and spinal instruments to target tissues to minimize collateral damages to tissues [13].
However there is a steep learning curve for spine surgeons in endoscopic spine surgery [6]. This is confounded by complexity of spinal procedures and generally higher risk procedures in spine surgery. There is a lack of training in handling of equipment which are specifically designed for endoscopic spine surgery during residency coupled with the paucity of endoscopic spine surgeon in the population of spine surgeon are some of the issues which limits the spread of practice in endoscopic spine surgery [52]. In this editorial, we highlight the challenges in the early phase of endoscopic spine surgery and the steps to mitigate complications and improve outcomes during this difficult learning curve period. We use the mnemonics BIPORTALS, namely basic medium, instruments/visualization, placement of portals, outflow of fluids, route of approach, technical/anatomical challenges, location of lens and light source and start point to work to highlight the differences among the 3 common types of minimally invasive and endoscopic surgeries and discuss strategies to overcome the differences and challenges in the different types of surgeries. A ladder of progression of endoscopic spine surgery is proposed to progressively ease into the practice of endoscopic spine surgery.
1. Basic Medium
Tubular microscopic surgery utilizes air base medium by exposure of a tract to the sterile surgical environment from skin to the target anatomy filled with air [3]. This is a familiar environment for most surgeons as we are used to open procedures during surgical training. The main disadvantage is the requirement of frequent suction and the use of bipolar and monopolar diathermy for hemostasis and visualization [17]. It is debatable that exposure of wound to airflow in operating room environment might increase risk of infection. The use of laminar airflow has shown to decrease the risk of infection [40].
Uniportal and biportal endoscopic surgeries are performed under water based medium. Constant irrigation with saline with/without antibiotic solution has the advantages of provision of clarity of visualization by decreasing bleeding from capillaries, constant elimination of bone dust and loose soft tissue by the outflow current and potential decrease in infection risk [48,50]. The use of water medium however brings the risk of water pressure related complications to central nervous systems. Water based complications such as soft tissue edema, cerebral edema, post-operative neck pain [22]. Recently, Kang et al showed that an irrigation pressure of mean 30 mmHg is safe for irrigation based surgeries [19]. It is a good practice to lower the pressure at the completion of the procedures to allow good hemostasis with radiofrequency ablation.
2. Instruments and Visualization
1) Retractor Tube, Endoscope and Working Instruments
In tubular surgery, soft tissue adjacent to bony target is retracted by accurate placement of tubular retractor under fluoroscopic guidance. It is imperative for the accurate placement of tube to minimize soft tissue damages and decrease operative time. Preoperative plans are made for accurate placement of the tubular retractor [36]. Microscope is used for visualization. These equipment are generally available in most hospitals with spine and neurosurgical practice. Spine surgeons are generally familiar in handling the microscope, though dedicated training might be required to be competent in tubular surgery [42].
Uniportal ESS on the other hand requires specific endoscope and dedicated instruments in each type of endoscopic approaches. In transforaminal endoscopic lumbar discectomy (TELD), a higher degree ocular angle endoscope(25-35 degree) than interlaminar endoscope (10-20 degrees) is required to visualize tissue in a limited working space with endoscope often docked at an angle to the target disc [47,58]. The endoscope is longer than interlaminar endoscope as the skin incision made tends to be further from target disc. A longer transforaminal endoscope might be required in patients with high body mass index [20].
The instruments in transforaminal approach are correspondingly longer than those used in interlaminar discectomy endoscope. Wider working channel dimensions endoscope and its correspondingly larger dimension endoscopic instruments are provided for lumbar endoscopic unilateral laminotomy with bilateral decompression (LEULBD) designed to use for stenosis cases [23,37]. Biportal ESS on the other hand uses mostly open spine general equipment and orthopaedic arthroscope. As the surgeon tends to stand in the same position throughout the procedure, typically using his dominant hand to handle power tools and sharp instruments, there are modified equipment such as rotatory Kerisson rongeurs, semi/full tubular working portal retractor, angled osteotomes which facilitate BESS/UBE working through a small working portal [18]. Unlike knee and shoulder arthroscopy which tends to use angled degree scope, 0 degree scope is popular in BESS/UBE. More than 1 working instruments for example retractor and Kerrison rongeur can be used simultaneously in tubular and BESS/UBE while in uniportal ESS, there is space only for one instrument to pass through working channel at one time. Typically working beveled tip cannula can be used a substitute for neural or soft tissue retraction in uniportal endoscopic spine surgery.
As working instruments are inserted into patient’s body from a separate portal, it is important to pull back endoscope to visualize the entry of the working instruments for beginners. Non visualized insertion of instruments can lead to instruments causing damages and injuries.
3. Endoscopic Drill and Energy System
In microscopic surgery, an air based angled handle drill of standard length can be placed through the tube. Bone dust is washed away by assistant providing irrigation and suction. The choice of drill bits is according to surgeon’s preference. Side cutting burr is a popular choice in tubular surgery as it is efficient and the blunt tip decrease chances of injury to the neural elements.
In both Uniportal and Biportal ESS, the endoscopic drill needs to be water resistant, compatible in water based surgical environment [54,56]. Biportal ESS uses standard working length drill is similar to microscopic surgery, a straight handle is recommended and the surgeon holds the drill on right hand only most of the time during surgery. In Uniportal ESS, the length and dimension of the drill varies according to the dimension of the working channel of each type of endoscope. The drill has a relatively longer handle and hence the rotating burr is of a long distance away from the motor, careful manipulation of the drill is important to prevent injuries to neural elements. Diamond burr is popular for both uniportal and biportal ESS as it generates heat and helps in hemostasis during drilling. Bone dust is constantly washed away by endoscopic irrigation.
The technique in handling uniportal and biportal ESS is very different. In Uniportal ESS, drilling is done collinear to the scope view, surgeon has to manipulate by rotating the endoscope to have better visualization of the tip of the drill and tissue it is working on. The drill is held with dominant hand with the non-dominant hand holding the endoscope and supporting the drill with a finger (Figure 1A). In Biportal ESS, the drill is held by dominant hand only without the support of non-dominant hand from a separate working portal (Figure 1B). The viewing scope visualize the tip of the drill at an angle of 30-90 degrees.
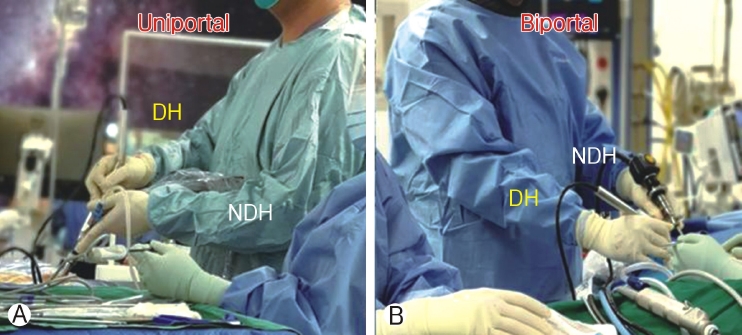
Ⓐ: Handling of Uniportal endoscope with non‐dominant hand maneuverers the endoscope and dominant hand (DH) holding the working instruments place through the working channel. Ⓑ : Nondominant hand(NDH) holding the endoscope from viewing portal and dominant hand holding working instruments placed through a separate working portal.
Figure 1A: Handling of Uniportal endoscope with non-dominant hand maneuverers the endoscope and dominant hand (DH) holding the working instruments place through the working channel. Figure 1B: Non-dominant hand(NDH) holding the endoscope from viewing portal and dominant hand holding working instruments placed through a separate working portal.
Due to limited working space available, care must be taken to prevent drilling of the lens which would damage the endoscope. The trick is to past point the drill during drilling, keeping the drill at 10-11 o’clock position during initial drilling at a limited working space in Biportal ESS (Figure 2A). While in Uniportal ESS, endoscopic drill that should be completely stopped and at rest before retraction back into the working channel of the endoscope (Figure 2B). During uniportal endoscopic drilling, surgeon needs to keep the drill collinear with the endoscope which in turns is collinear with working retractor tube, any bending forces on the drill cause drill breakage due to long lever arm of the endoscopic drill, while any bending forces on the endoscope cause breakage of the endoscopic lens.
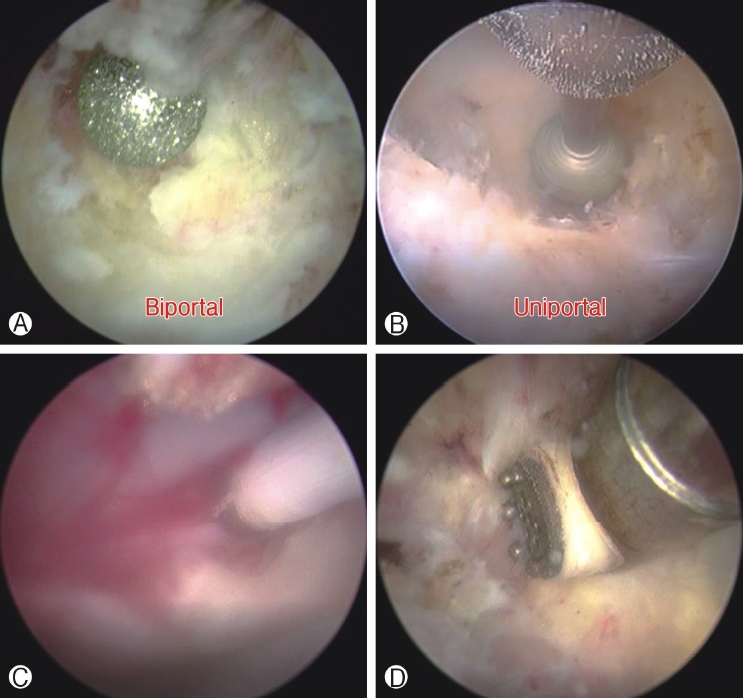
Ⓐ : Endoscopic drill is visualized at 10‐11 o’clock position of the endoscope past pointing the endoscope during initial drilling in a limited working space in biportal endoscopic spine surgery to prevent damages of endoscope to prevent lens damages. Ⓑ : In uniportal endoscopic spine surgery endoscopic drill is in forward position relative to endoscope. Endoscopic drill that should be completely stopped before retraction back into the working channel of the endoscope to prevent lens damages. Ⓒ: Low energy radiofrequency ablation is compatible with work near dura region for hemostasis which is applicable for both uniportal and biportal endoscopic spine surgeries. Ⓓ: Higher energy radiofrequency ablation can be applied to soft tissue dorsal to deep layer of ligamentum flavum to allow more efficient soft tissue dissection, different length and dimensions are applicable for high radiofrequency ablation applicators in uniportal and biportal endoscopic spine surgeries.
Electrical energy in central nervous system in general and spine in particular are of a different energy setting as compared to limb orthopaedic surgery. Surgeons who are doing each of these techniques have to familiarize themselves with the limitations of the technique and derive a workflow to overcome these challenges. In both uniportal and biportal ESS, low energy radiofrequency ablator is compatible with work near dura region is generally used for hemostasis (Figure 2C). High energy radiofrequency ablator is optional in uniportal ESS, often used in stenosis decompression due to higher workload of bony decompression and corresponding soft tissue dissection. High energy radiofrequency ablator is however strongly recommended as a requirement for biportal ESS as there is much work in soft tissue dissection and for triangulation and creation of working space in the sublaminar region deep to spinal muscles but dorsal to the deep ligamentum flavum (Figure 2D). In this specific zone of interlaminar region and sublaminar space, the energy setting range is very specific, higher than the energy required in radiofrequency ablation for spine pain procedures but lower than limb orthopaedic surgery setting. If energy is too high it can cause a phenomenon which is similar to Electric Convulsive Therapy (ECT) which can cause patient to be in a stupor state or worst scenario of cardiac complications such as asystole [45]. We do not recommend use of arthroscopic setting use in knee and shoulder to be use in spine [51]. While an energy setting which is too close to low energy radiofrequency ablation may not be efficient in soft tissue dissection in a layer dorsal to ligamentum flavum. It is important to check whether the energy machine used is specific for spinal water based surgery with both compatible high and low energy radiofrequency ablation is available [4].
Figure 2A: Endoscopic drill is visualized at 10-11 o’clock position of the endoscope past pointing the endoscope during initial drilling in a limited working space in Biportal Endoscopic Spine Surgery to prevent damages of endoscope to prevent lens damages. Figure 2B: In uniportal endoscopic spine surgery endoscopic drill is in forward position relative to endoscope. Endoscopic drill that should be completely stopped before retraction back into the working channel of the endoscope to prevent lens damages. Figure 2C: Low energy radiofrequency ablation is compatible with work near dura region for hemostasis which is applicable for both uniportal and biportal endoscopic spine surgeries. Figure 2D: Higher energy radiofrequency ablation can be applied to soft tissue dorsal to deep layer of ligamentum flavum to allow more efficient soft tissue dissection, different length and dimensions are applicable for high radiofrequency ablation applicators in uniportal and biportal endoscopic spine surgeries.
Choice of Biportal and Uniportal ESS. Both biportal and uniportal ESS techniques have their merits and disadvantages (Table 1). It is imperative for the surgeon to understand his local community facilities and equipment support to see the feasibility of his ESS practice.

Differences in tubular microscopic spine surgery, Uniportal endoscopic spine surgery and biportal endoscopic spine surgery
In Uniportal ESS, the equipment required is generally specifically designed for use in Uniportal ESS as there are various different dimensions and requirement for the different approaches. In transforaminal endoscopic lumbar discectomy, the limitation of space in Kambin’s safety triangle and the relatively long distance from the skin surface means there is a need for a long and slim endoscope with a correspondingly small working channel as compared to interlaminar approaches. These different dimensions translate into purposed built endoscopic equipment sizes and types of endoscopic equipment. As these equipment are specifically designed for spinal endoscopy, they can be expensive to obtain and make available to the surgeon’s local community. Biportal ESS uses ordinary or lightly modified general spinal equipment, arthroscope can be available from general orthopaedic practice with slight modification to fit Biportal ESS practice. There is high reliance of suitable and safe energy source for spine to provide hemostasis in the form of suitable energy radiofrequency ablation. These energy equipment may not be available locally in surgeon’s community. As these energy instruments are single use, this can add to the total cost of the procedure.
Before a surgeon embarked a journey for fellowship in endoscopic spine surgery, he should check with the local vendors and community for availability of these specialized endoscopic equipment to make decision on where he should focus on training. As it is costly to set up the equipment required for endoscopy, sufficient volume of cases is needed to make a business case for funding when presenting to hospital management [39].
1) Placement of Portal(s), Outflow of Fluids, Route of Approach, Location of Lens/light Source and Start Point to Work
In microscopic tubular spine surgery, the retractor tube is fixed to the table after confirmation of position under fluoroscopic guidance. Changes in position is possible with tilting of surgical table and microscope typically with fluoroscopic guidance. The retractor tube is collinear with microscope. A typical docking spot is spinolaminar junction or laminofacet junction. The spinous process anatomy can affect docking position (Figure 3). Soft tissue is dissected after docking to expose the underlying lamina. Surgeons do not encourage frequent changing of position of the working tube as it consumes time in adjustment of surgical table, microscope and fluoroscopy; each change in docking position will require some soft tissue dissection with diathermy [11].
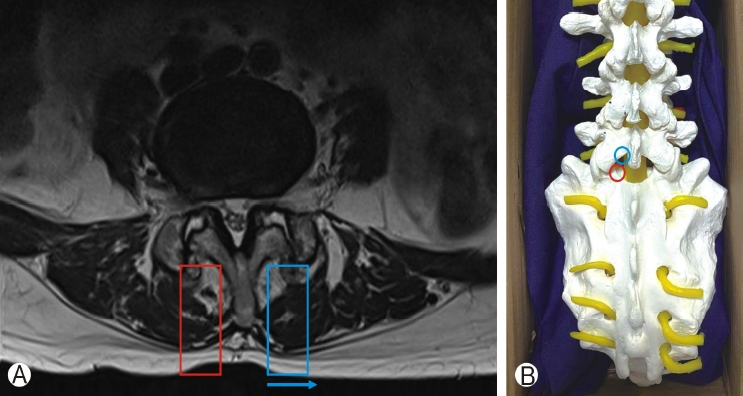
Ⓐ: Spinous process is deviated to the left side. For tubular retractor placement, spinous process deviation will displace the tube (blue rectangle) towards the left side (blue arrow) as compared to the right side(red rectangle). Ⓑ: 2 typical fluoroscopic guided docking sites for uniportal endoscope and microscopic tubular retractor. Spinolaminar junction(black circle) and laminofacet junction also known as “v”point (red circle). Biportal endoscopic spine surgery convertges the viewing endoscope and working instruments to spinolaminar junction (black circle) in the initial period of positioning under fluoroscopic guidance.
Figure 3A: Spinous process is deviated to the left side. For tubular retractor placement, spinous process deviation will displace the tube (blue rectangle) towards the left side(blue arrow) as compared to the right side (red rectangle). Figure 3B: 2 typical fluoroscopic guided docking sites for uniportal endoscope and microscopic tubular retractor. Spinolaminar junction(black circle) and laminofacet junction also known as “v”point (red circle). Biportal endoscopic spine surgery converges the viewing endoscope and working instruments to spinolaminar junction (black circle) in the initial period of positioning under fluoroscopic guidance.
Uniportal ESS docked at key bony landmarks or defined safe working space. Once docking is confirmed with aid fluoroscopy, the mobility of endoscope is excellent around the docked region. In TELD, there are numerous approaches described in the literature docking on various landmarks. The 2 commonly described docking landmarks are Kambin’s triangle in the (inside-out technique) and ventral lateral margin of facet joint in foraminoplasty (outside-in or mobile outside-in technique). Due to the constrain of working space, a higher angle of endoscope (25-35 degrees) are needed to visualize the working space (Figure 4A-C) [28].
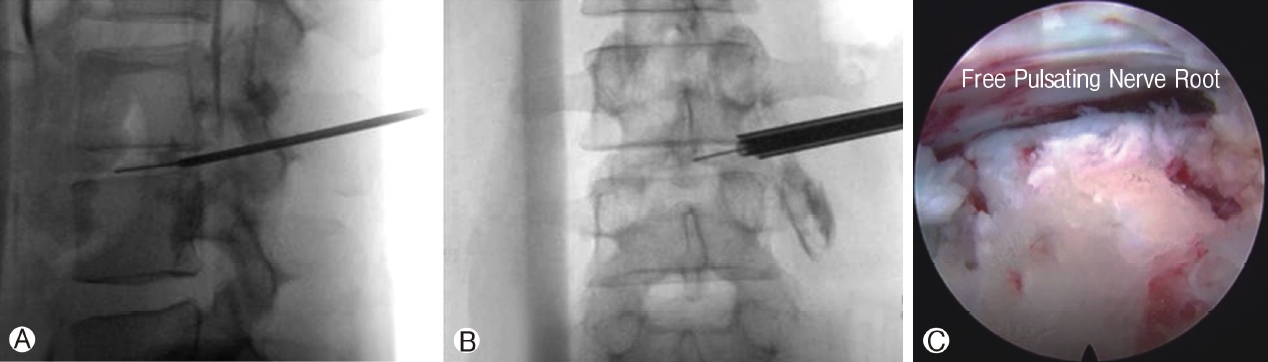
Ⓐ: Docking of dilator and guidewire on the distal dorsal aspect of intended intervertebral disc. Ⓑ: Mobile outside in technique with dilator and working channel placed at the entrance of foramen at the midline of pedicle on the disc space. Ⓒ: Higher degree endoscope of 30 degrees in this case to visualize the free pulsating traversing nerve root.
Figure 4A: Docking of dilator and guidewire on the distal dorsal aspect of intended intervertebral disc. Figure 4B: Mobile outside in technique with dilator and working channel placed at the entrance of foramen at the midline of pedicle on the disc space. Figure 4C: Higher degree endoscope of 30 degrees in this case to visualize the free pulsating traversing nerve root.
In IELD on the other hand, docking is often directly on the superficial portion of ligamentum flavum (Figure 5A and B). in line with the prolapse disc on lateral view and on the shoulder or axilla region of the neural elements on anteroposterior view [47]. (Figure 5C).
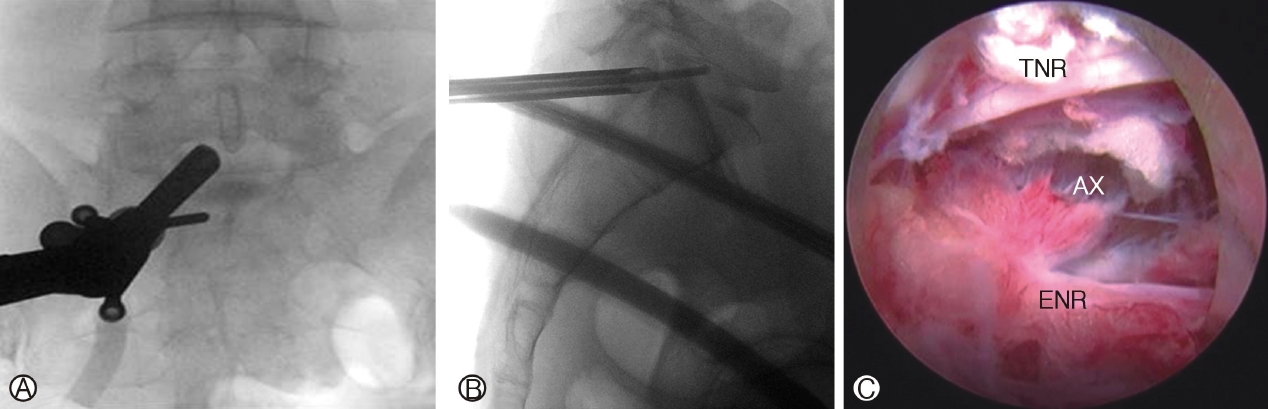
Ⓐ: Interlaminar Endoscopic Lumbar Discectomy, endoscope placed medial to the facet joint of left L5/S1. Ⓑ: Route of advancement for a caudally migrated disc through the axilla region of neural elements, annular defect at the distal portion of the disc paracentral region. Ⓒ : Axillary route (Ax) approach to disc between traversing nerve root (TNR) and exiting nerve root (ENR). The shoulder approach places endoscope on the lateral margin of the shoulder of the exiting nerve root.
Figure 5A: Interlaminar Endoscopic Lumbar Discectomy, endoscope placed medial to the facet joint of left L5/S1. Figure 5B: Route of advancement for a caudally migrated disc through the axilla region of neural elements, annular defect at the distal portion of the disc paracentral region. Figure 5C: Axillary route (Ax) approach to disc between traversing nerve root (TNR) and exiting nerve root (ENR). The shoulder approach places endoscope on the lateral margin of the shoulder of the exiting nerve root.
In LEULBD, the docking point of spinolaminar junction and laminofacet junction (V-point) are the commonly described anatomical landmarks. The endoscope used in interlaminar approaches is comparatively lower angle of 10-20 degrees [23]. In higher lumbar levels, the interlaminar space is narrow and it may not be compatible to relatively large uniportal endoscope and retractor dimensions make it difficult to land on the medial aspect of the laminofacet junction (V-point). A smaller endoscope can be considered after initial bone work with stenosis scope to provide better visualization of higher lumbar level interlaminar space. Preoperative planning on where to dock is imperative for TELD, IELD and LEULBD as the endoscope. After skin and fascia incision, the working retractor is directly docked on area of concern and endoscopic surgery is performed around the docked region. ESS is highly magnified and focus on the region of the pathological anataomy, despite high mobility of uniportal endoscope, erroneous docking would result in significant disorientation and unnecessary collateral soft tissue damages both leading to increase in operative time and risk of complications and/or incomplete decompression. Verification with fluoroscopy can help in orientation, use of navigation can help to do real time orientation while doing endoscopic procedure [12]. Biportal ESS has a few considerations in terms of position for docking. (1) Viewing portal (VP) is 2-3 cm away from working portal (WP) to allow space for both endoscope and working instruments to meet in the targeted anatomical region. (2) VP and WP should not be too cephalad in position which would lead to excessive and unnecessary amount of bony decompression. (3) VP and WP should not be too caudal in position which would be inefficient for working instruments to reach the cephalad ligamentum flavum attachment. (4) VP and WP should not be too lateral which would lead to excessive and unnecessary amount of ipsilateral facet dissection and difficulty in reaching contralateral side. (5) In patients with high body mass index, a more lateral entry point is required to visualize contralateral side. (6) For a right handed surgeon, the right side has a more caudal docking point than the left side to limit the amount of cephalad laminotomy for working instruments to gain access to the contralateral side. Position of BESS and UBE are well described in several literature [7,16,38]. Most articles describe docking point for decompression to be on spinolaminar junction. The position is not fixed and the surgeon can vary the position with considerations made as described above. If surgeon has any doubt about his endoscopic position during surgery, he should perform intraoperative fluoroscopy to clarify the position of his endoscope.
The water inflow and outflow is provided from one port and one scope in uniportal ESS. Obstruction in outflow cannula can lead to blurring of the endoscopic vision. Safety in water pressure and constant inflow and outflow of fluid is paramount to clarity of surgical field and safety to central nervous system. A sustained high pressure of water flow can lead to undesirable complications such as neck pain, cerebral edema, soft tissue edema, hydrothorax, hydroperitoneum [22]. These complications are rare and is seen mainly in case reports due to a combination of disorientation, violation of soft tissue and high water pressure. While a low water pressure will compromise clarity of vision. Outflow management is important, without proper outflow of fluid, the debris and blood does not wash out and impedes endoscopic vision. An erroneous increase in inflow pressure in an attempt to improve visualization in a blocked outflow will lead to high pressure in spinal region beyond the safety acceptable for ESS. Recent article showed a pressure of 30 mmHg is acceptable and safe for biportal endoscopic spine surgery [19]. The authors used similar pressure setting for uniportal endoscopic surgery.
2) The location of Lens and Light Source and the Start Point to Work is a Key Conceptual Difference between the 3 Types of Minimally Invasive Surgery
In microscopic tubular surgery, lens and light source is located in the microscope which is outside the patient’s body at the eye level of the surgeon and assistant. Any tissue from the start point of work from skin to the area of interest is retracted/dissected away to decompress the neural elements and allow visualization at the level of microscope. Some level of soft tissue collateral damage is necessary to allow visualization of tissue from skin to lamina region of the target level. In uniportal and biportal endoscope, the start point is directly on the docked region under the muscle and at times even beyond lamina or facet in discectomy cases. The lens and light source is at the tip of endoscope deep in the patient’s body right at the target. This decrease collateral damage in exposure of the tissue for visualization. In biportal ESS, some soft tissue dissection is required for triangulation of VP and WP, which can be assisted by the combination of electric energy system of plasma coagulator, radiofrequency ablator and shaver.
4. Technical and Anatomical Challenges
Most of the residents and fellows are used to visualize anatomical structures under microscope in their training. Visualization is more 3D in microscope while 2D in endoscope. They are often given hands on opportunities during their training. Such hands on opportunities are less common in ESS practice. As endoscopic spine surgery (ESS) is a new trend of spine surgery practice, there is a low proportion of endoscopic spine surgeon in most orthopaedic and neurosurgical residency and fellowship training centres. There is generally a lack exposure of ESS techniques during training period of the junior surgeons. There are various ways to learn ESS, such as partaking an endoscopic spine fellowship or observership, participation in cadaveric and dummy workshop. The key limitation in cadaveric workshop is the lack of bleeding and hemostasis required during cadaveric practice. Korean Minimally Invasive Spine Society had recently recognized and accredited some of the international fellowship training centres for endoscopic spine surgery, this is helpful in aspiring ESS fellows to make an informed decision on which training centre is suitable to his ESS interest. Several hospitals in different countries also advertised for international endoscopic fellowship [52].
There are a few inherent technical challenges in visualization and instruments usage in endoscopic surgery. In microscopic surgery, there is collinear zero degree visualization, magnification can be adjusted by microscope setting. Triangulation of instruments is easy as the instruments are seen directly from skin level to target anatomy by microscope. Angulated instruments help to prevent obstruction of microscopic vision. Working space is created by the working tube and soft tissue dissection, we can engage an assistant surgeon for retraction, suction and suction. An experienced surgeon can be the assistant and partake in majority of the procedures. Multiple instruments can be used simultaneously during procedure [11]. In Biportal ESS, 0 degree visualization is possible with zero degree scope which can be less disorientating to surgeons. In uniportal ESS, an angled visualization (10-30 degrees) is necessary to visualize the tip of instruments which is working in front of the endoscope. Magnification in both uniportal and biportal ESS is adjusted by pushing the endoscope deeper for more magnification and shallower for lower magnification. However, a helicopter point of view is not possible from uniportal ESS while biportal gave a broader helicopter point of view when instruments are introduced from working portal as viewing portal is independent from working portal. This freedom of viewing and instruments usage comes with a price of possible neural injuries or soft tissue damages by working instruments while scope is setting up a “meeting position “with the instrument. Working port retractor helps to guide instrument safely to the target to prevent inadvertent injuries to neural elements and also create a channel for outflow of water [7].
Creation of working space in Biportal ESS is necessary for scope and instruments to safely navigate and work in contralateral sublaminar region this typically required a working distance of 13 mm between bases of spinous process of cephalad and caudal lamina to accommodate a 8 mm endoscope and 5 mm instruments A working space is required for uniportal ESS for 10-13 mm according to size of stenosis scope to allow the scope to pass through the base of spinous process to contralateral side. Inadequate working space can lead to scope and instruments impingement and possible inadequate decompression. With sufficient working space, there is great mobility of endoscope. The key difference in working instruments mobility is significant among the 3 types of minimally invasive surgery. There is slight limitation of instruments number, mobility and direction by the tube in tubular surgery, very limited instruments mobility in uniportal ESS as all instruments are inserted through working channel and no limitation in numbers of instruments, ports or direction in biportal surgery as viewing and working portals are independent. This limitation is extended to the number of assistant surgeons as well. In microscopic surgery, one assistant is needed due to limited working space in tube and limited viewing lens in microscope limits to one assistant most of the time. In uniportal ESS, there is neither space nor opportunity for an assistant to aid in the surgery, neural and soft tissue retraction is generally done by beveled working retractor rotating with the opening away from neural structures. In Biportal ESS, one can work with or without assistant according to the surgeon’s choice. Some prefer to use working retractor through working channel and scope retractor to retract neural tissue without assistant, while others engage an assistant to hold retractor through the working portal. All observers, assistant and nursing staff can visualize the steps of surgery through the TV output monitor in endoscopic surgery. Despite the differences in technique, the clinical results are comparable in the 3 techniques with better outcomes in early postoperative period in endoscopic surgery [15,33].
1) Selection of Cases and Ladder of Progression of Endoscopic Surgery
There is a steep learning curve in endoscopic spine surgery. ESS surgeons need to be prudent in selection of cases in the early stages of development of ESS practice. We proposed a stepwise progression ladder in the development and selection of cases of ESS for consideration to maximize positive outcomes and minimize complications (Figure 6). There are key challenges and rationale in the construction of this ladder.
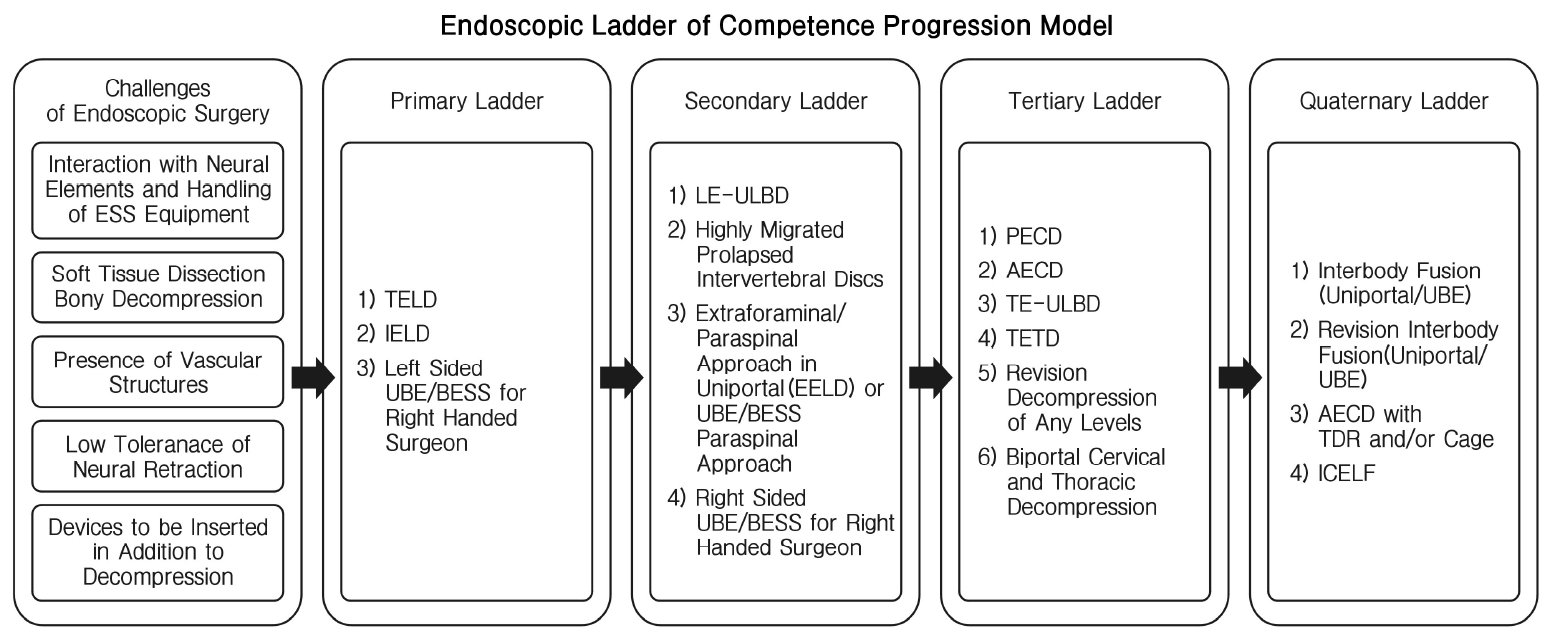
Endoscopic ladder of competence progression model. transforaminal endoscopic lumbar discectomy (TELD), Interlaminar endoscopic lumbar discectomy (IELD), Unilateral biportal endoscopy (UBE), Biportal endoscopic spine surgery (BESS), Lumbar endoscopic unilateral laminotomy bilateral decompression (LE‐ULBD), Extraforaminal endoscopic lumbar discectomy (EELD), Posterior endoscopic cervical decompression (PECD), Anterior endoscopic cervical decompression (AECD), Thoracic endoscopic-unilateral kaminotomy bilateral Decompression (TE‐ULBD), Transforaminal endoscopic thoracic discectomy (TETD), Total disc replacement (TDR), Interlaminar contralateral endoscopic lumbar foraminotomy (ICELF).
2) Interaction with Neural Elements and Handling of Endoscopic Equipment Considerations for Primary Ladder of Competence Progression Model
TELD as a procedure has several technical nuances to be recommended as the first ESS surgery for uniportal ESS surgeon. The presence of ventral portion of facet and foraminal ligament served as a good protection shield to prevent inadvertent neural injuries. The caveat is that reaming or drilling should not go beyond the medial portion of pedicle on AP fluoroscopic view. Inside out technique is safer than outside in technique for traversing nerve root but tends to have higher rate of dysesthesia in patients with narrow foramen [28]. IELD can be generally divided into 3 routes to approach the disc. First is the approach to ligamentum flavum which can be split, cut or resected, second is the approach to neural elements which can be shoulder approach or axilla approach, third is the handling of the disc with sequestrectomy, discectomy with or without annuloplasty [25,34,35]. There is more interaction with neural elements and more delicate handling of working retractor required in IELD as compared to TELD, however there is familiarity of the approach as most surgeons are well trained in posterior approach to the spine during residency.
For most right sided surgeon, left sided L4/5 Biportal ESS surgery is a good start for the first few cases of endoscopic decompression. Left sided approach would allow the instruments to approach the cephalad lamina in a more natural trajectory under vision of left viewing port. Multifidus muscle can be more easily retracted in left sided UBE/BESS, minimizing soft tissue dissection and bleeding. The view for beginner ESS surgeon is more familiar in left side approach when viewing port is held on the left hand in left sided approach [6].
3) Soft Tissue and Bony Decompression and Presence of Vascular Structures Considerations for Secondary Ladder of Competence Progression Model
For LEULBD, there is a need for more bony resection by either endoscopic drill in outside in technique popularized by Kim and Wu et al [23], or usage of Kerrison in Inside Out technique popularized by Lim et al [37], there is a need to handle more bony elements of the spine, more soft tissue dissection with energy system and the need for endoscope to advance to contralateral side for decompression. All these technical steps present a steep learning curve for ESS surgeon.
Highly migrated disc presents a challenging scenario for both TELD and IELD approaches. There is a risk of inadequate decompression if insufficient bony and soft tissue resection is done to reach the target migrated disc. Often more reaming or endoscopic drilling is required [21].
In Extraforaminal or paraspinal approach, the approach is facing directly on the exiting nerve root with its associated radicular artery. The proximity of radicular artery is a challenge to new ESS surgeon, as injury to radicular artery can lead to bleeding which is difficult to control [8,10]. As this is an unfamiliar surgical anatomy to new ESS surgeon, disorientation with excessive dissection beyond the intertransverse membrane can lead to hemoperitoneum or hydroperitoneum [2].
Right sided UBE/BESS for right handed surgeon presents with a different angle of vision with the viewing endoscope looking at the lamina from caudal to cephalad and bottom up direction with working instrument coming from the dorsal aspect of lamina. Surgeon tends to make the 2 portals in a slightly more caudal direction to decrease the amount of bone lamina resection required for the working instruments to enter interlaminar space [6,8].
4) Low Tolerance of Neural Retraction for Tertiary Ladder of Competence Progression Model
Surgeries at cervical and thoracic region has added risk as these are cord level surgeries. Spinal cord is intolerant of any sort of manipulation and retraction [55,57]. This is confounded further by the presence of important vascular structures such as vertebral artery in the foramen transversarium and aorta and segmental arteries in thoracic spine. These vascular structures injuries is catastrophic, which can lead to risk of significant morbidity and mortality. In AECD, additional care is given to prevent esophagus and trachea injuries during approach [5]. Hence both anterior and posterior endoscopic cervical and thoracic decompression with uniportal and biportal ESS approaches are advanced level ESS surgeries.
Revision cases tend to have dense adhesion with increased risk of inadvertent dura tear, manipulation of the neural elements is challenging and hence we advised ESS surgeon to be more experienced in ESS and handling of dura tear using endoscopic technique before performing revision endoscopic decompression [46].
5) Devices to be Inserted In Addition to Decompression for Quaternary Ladder of Competence Progression Model
Interbody fusion is challenging in many ways. More bone resection is required with endoscopic drill or osteotome during the procedure. End plate preparation needs to be precise without injuries to end plate which increased risk of subsidence while inadequate preparation increased risk of pseudoarthrosis. Cage insertion under a narrow endoscope channel is often not possible, modified technique is required with modified instruments or specialized cage glider might be necessary. Cage can be placed either under direct endoscopic vision or fluoroscopic guided insertion with endoscopic inspection after cage inserted in the interbody space. Fine adjustments can be done after cage is inserted under endoscopic vision [31,56]. Disc replacement and cage insertion in cervical spine and interbody cage insertion in lumbar spine under endoscopic procedures are new surgical techniques in the literature under experts’ hands. More data is being evaluated for the evolution of these techniques.
Interlaminar contralateral approach has been described in the literature, however the use of a small caliber endoscope through the contralateral foramen from the contralateral side can be disorienting to new ESS surgeon. This anatomical approach is not often explored under training hence the authors felt that it is part of an advanced technique to be done in later stages of ESS surgeons’ career [26,53].
6. Potential Complications of Endoscopic Spine Surgery and Management Strategies
The range of complications for uniportal and biportal endoscopic spinesurgeries are similar as the surgeries are performed in the same anatomical region. While devastating injury such as nerve root injury and paralysis are uncommon in experienced surgeons, common complications such as dura tear, water irrigation related neck or head discomfort, postoperative hematoma and postoperative dysesthesia are common. Fortunately, these complications often can be treated endoscopically and/or with conservative treatment.
1) Dura Tear
Dura tear rate is approximately 2.7% (0-8.6%) in endoscopic spine surgery [27,43,59]. The rate is higher in decompression and fusion than discectomy. Early learning curve period tends to have a higher dura tear rate. Dura tear of more than 1cm should be considered for conversion to open surgery. For dura tear of 1 cm and below, several treatment strategies had been proposed. Use of adjuncts such autologous muscle or fat graft in combination with fibrin glue or fibrin sealed collagen sponge is good and safe method for management of dura tear [41]. Use of tachosil and gel foam construct is popular [27]. Endoscopic direct repair by stapler anastomoclip and/or sutures are possible [14,49].
2) Water Irrigation Related Neck and Head Pain:
Postoperative Raise in Intracranial Pressure Raised in intracranial pressure secondary to high endoscopic saline inflow can lead to cerebral edema and seizure [9,44]. Prevention is key to to prevent such complications. Ideal water pressure used is around 30 mmHg (25-50 mmHg), usage of 75-80 mmHg should be limited to less than 30 minutes if needed to prevent neck pain, and postoperative headache.
6) Hematoma formation
Postoperative hematoma is a dreadful complication of all spine surgeries, endoscopic spine surgery is of no exception. Kim et al showed a 24.7% rate of hematoma in biportal endoscopic spine surgery with only 1.2% developed neurology and required revision surgery because of hematoma [32]. Careful hemostasis is key to prevent postoperative hematoma formation. In the presence of an increase in neurological deficit of bilateral limbs postoperatively, a low threshold for revision decompression and drainage of hematoma is necessary.
4) Postoperative Dysesthesia
Postoperative dysesthesia is a common complication in surgery involving foraminal diseases, including lumbar foraminal or extraforaminal herniated nucleus pulposus (HNP). Minimal dorsal root ganglion retraction is key to preventing postoperative dysesthesia. Kim et al showed interlaminar contralateral approach has less postoperative dysesthesia rate as compared to transforaminal endoscopic lumbar discectomy [24]. Preoperative evaluation is key in transforaminal endoscopic lumbar discectomy and foraminotomy surgeries. Narrow foramen or duplicate/conjoined exiting nerve root are signs increased risk of postoperative exiting nerve root dyesthesia. Endoscopic surgeon should either choose an alternative endoscopic strategy or careful superior articular facet decompression before docking the working cannula in an already narrowed foramen [1].
Summary and Conclusion
Overall despite the technical, administrative and clinical challenges in endoscopic surgery, its result shows positive impact on patients’ outcome. A fellowship or observership with an experienced endoscopic surgeon would be helpful in early stages of endoscopic spine surgery. Consent should be given for possible open conversion from endoscopic spine surgery during early learning period. Understanding the inherent differences in endoscopic techniques to match the requirement of surgeons’ patients and institution can help to overcome the steep learning curve of endoscopic spine surgery.
Notes
Dr. PH Wu is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. There are no other potential conflicts of interest relevant to this article to declare.