The Efficacy of Second-Generation Cervical Spine Arthroplasty: A 5-Year Perspective
Article information
Abstract
Objective
The aim of this study was to determine the surgical outcomes of second-generation cervical arthroplasty in patients with symptomatic cervical disc degeneration at approximately 5 years of follow-up.
Methods
A retrospective study was conducted of consecutive patients who underwent elective cervical spine arthroplasty at our institution with the use of a second-generation cervical prosthesis. The inclusion criteria were: (1) patients aged 25 to 50 years, (2) patients undergoing primary cervical surgery, (3) a minimum follow-up of 5 years. Demographic, clinical, surgical, and radiological data were collected and analyzed for patients who met the inclusion and exclusion criteria.
Results
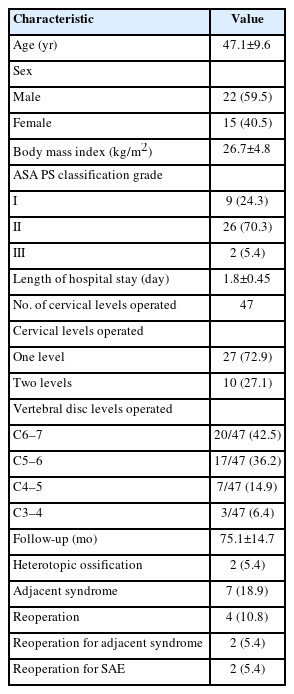
Thirty-seven patients were identified. The mean final follow-up was 75.1 months (range, 62.5–92.2 months). Statistically significant improvement was observed at the end of follow-up in the cervical disability index, neck pain, and radicular pain (p<0.001). In total, 89.1% of patients reported being satisfied or very satisfied. During follow-up, 4 patients required reintervention, including 2 for adjacent segment syndrome, 1 for possible implant failure, and 1 for a prevertebral collection. The mean mobility of the operated segment at 5 years was 8.1 (standard deviation, 5.91). Additionally, radiologically, 1 patient exhibited heterotopic ossification, and 5 patients had asymptomatic adjacent segment syndrome.
Conclusion
Promising outcomes are associated with second-generation cervical arthroplasty in the management of symptomatic cervical disc degeneration. The improvement in clinical scores, preservation of segmental mobility, and low incidence of complications such as heterotopic ossification and reintervention underscore the potential benefits of second-generation cervical arthroplasty.
INTRODUCTION
Symptomatic cervical disc degeneration is a leading cause of work disability, often resulting in neck pain, radicular arm pain, myelopathy, and altered cervical spine biomechanics [1,2]. Published studies indicate an increasing trend in cervical surgery for this reason in the coming years, especially in the population aged between 45 and 54 years [2,3].
Anterior cervical discectomy and fusion (ACDF) remains the surgical treatment of choice for degenerative cervical disc disease [4]. Unfortunately, fusing the affected segment not only decreases movement at the fused level but also has an adverse biomechanical effect on adjacent discs, which essentially have to compensate for the loss of natural movement of the fused cervical segment [5]. Hilibrand et al. [6] found that symptomatic disease of the adjacent segment can affect more than a quarter of all patients within 10 years following ACDF. Lee et al. [7] found that after ACDF, secondary surgery in adjacent segments occurred at a relatively constant rate of 2.4% per year (95% confidence interval [CI], 1.9–3.0). Kaplan-Meier analysis predicted that 22.2% of patients would require reintervention in adjacent segments at 10 years postoperatively [7]. To avoid this risk, as well as others such as pseudoarthrosis, an alternative treatment emerged in the 1990s, cervical disc arthroplasty (CDA), with the aim of maintaining movement in the operated cervical disc [8,9].
While the number of CDA performed has increased, it has been reported that spine surgeons perform nearly 100 times more ACDF than CDA [8]. In their study of 167 patients undergoing ACDF, Auerbach et al. [10] found that 43% of patients would have had indications for CDA. They concluded that a significant number of patients currently undergoing ACDF could benefit from CDA [10].
Although cervical arthroplasty presents some drawbacks, the most common being heterotopic ossification (HO), implant failure, and bone loss [11,12]. Currently, a large amount of data in the literature report favorable clinical and radiological outcomes of cervical arthroplasty, especially in the short and medium term, and with first-generation cervical arthroplasties [11,13-20]. First-generation cervical arthroplasties were mechanical implants based on the experience of hip and knee replacement. These articulated designs involve a mechanical interface with a specific frictional torque but do not replicate the elasticity and impact absorption of the native disc [21-24].
Ideal cervical arthroplasties should provide triplanar motion: flexion and extension in the sagittal plane, lateral flexion in the frontal plane, and rotation and compression in the axial plane. "Second-generation" cervical arthroplasties are nonarticulating disc implants (deformable elastomeric core). Their structure is more complex with the aim of better reconstructing the physiological levels of impact absorption and flexion stiffness [25]. The aim of this study is to determine the surgical outcomes at 5 years of using second-generation cervical arthroplasties in the treatment of patients with symptomatic cervical disc degeneration.
MATERIALS AND METHODS
1. Study Design
A historical cohort of all patients consecutively undergoing scheduled cervical spine arthroplasty at our institution's spine unit using a second-generation cervical disc prosthesis (M6-C artificial cervical disc; Spinal Kinetics, Sunnyvale, CA, USA) was evaluated. Approval was obtained from the Fundació Assistencial Mútua Terrassa's (P/22-005) Ethics Committee to retrospectively review patients who had undergone this surgery between 2013 and 2016. Patients were identified through our institutional registry. The patients were informed that data and images concerning their cases would be submitted for publication, and they provided written informed consent. Informed consent was obtained from all participants.
2. Inclusion and Exclusion Criteria
• Inclusion criteria: (1) patients aged 25 to 50 years, (2) patients undergoing primary cervical surgery, (3) patients with a minimum follow-up of 5 years.
• Exclusion criteria: Patients with first-generation cervical disc prosthesis cervical, hybrid fusions or incomplete medical and radiological histories were excluded.
Patients were operated if they presented with symptomatic degenerative cervical pain or radiculopathy confirmed clinically and radiographically by magnetic resonance imaging. Patients were initially treated with nonsteroidal anti-inflammatory drugs and physiotherapy for a minimum of 6 months before considering surgery.
3. Description of Surgical Technique
1) Implant type
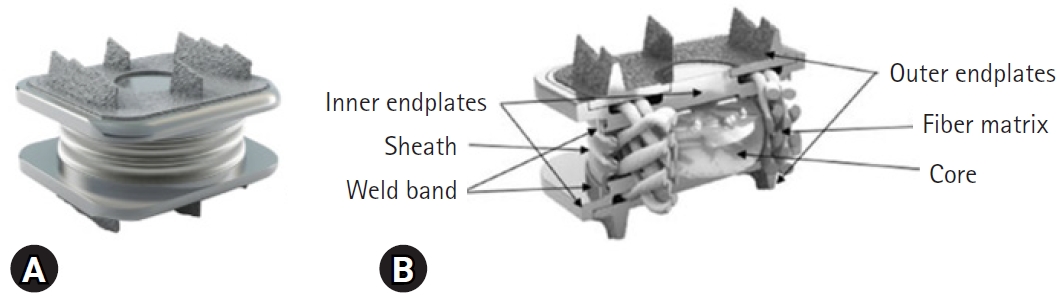
The design of the M6-C cervical disc prosthesis was first implanted in 2005. It was newly approved by the U.S. Food and Drug Administration on February 6, 2019. This cervical arthroplasty design incorporates a mobile artificial core made of polycarbonate urethane and a peripheral ring made of polyethylene (Figure 1) [23].
2) Surgical technique
Surgery was performed using a classic anterior approach to the cervical spine. Discectomy and decompression of neural elements were performed as in anterior cervical fusion, using the Caspar distractor. The appropriate size of the prosthesis was determined by inserting different templates. Fluoroscopy was used to establish the proper depth and height of the prosthesis. Once the final size of the prosthesis was decided, slots were created in the vertebral edges for the device's fins to adapt (Figure 2). Finally, the definitive prosthesis was inserted, and the wound was closed. Low-pressure drainage without vacuum was used for 24 hours postoperatively.
3) Follow-up
In patients who met the inclusion and exclusion criteria, demographic, clinical, surgical, and radiological data were collected and analyzed. Data were collected preoperatively, postoperatively, and during the follow-up period (1, 3, 6, 12, 24, 48, and 72 months postoperatively). For the final analysis, the last follow-up data for each patient were used.
Demographic and clinical data included: patient age at the time of surgery, sex, body mass index, American Society of Anesthesiologists (ASA) physical status classification, reason for surgery, type of pain (radicular yes or no), days of hospitalization, complications, reintervention. In addition, specific cervical spine functional scales such as the Neck Disability Index (NDI) and the visual analogue scale (VAS) of pain were collected. To assess clinical success (CCS), it was defined as the absence of supplemental surgical intervention (SSI) at the index level, encompassing revision, removal, reoperation, or supplemental fixation. Additionally, CCS required the absence of serious adverse events (SAEs) classified as device or index level procedure-related. Furthermore, a minimum improvement of 30/100 points in NDI scores compared to baseline, along with maintenance during the final follow-up, was considered indicative of CCS. Moreover, quality of life and patient satisfaction at the final follow-up were evaluated using a 5-point Likert scale (ranging from very dissatisfied to very satisfied).
Radiological analysis was performed using serial radiographic evaluation. All patients underwent a series of plain radiographs including anteroposterior, lateral, and flexion-extension views during their follow-up. Using the Cobb method on lateral cervical radiographs, cervical range of motion (ROM) at the treated level and globally for the entire cervical spine was measured, and disc height was also determined. Additionally, postoperative radiographs during follow-up were evaluated for the presence of HO.
4. Statistical Analysis
Descriptive statistics were used to present cohort characteristics. Categorical variables were described with their absolute values and percentages. Quantitative variables were presented by their measures of central tendency (mean and standard deviation). Statistical analysis to evaluate differences between preoperative variables and those at the end of follow-up used Fisher exact test for categorical variables and Student t-test for comparison of means. Differences with a p-value <0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
RESULTS
Thirty-seven patients were identified, comprising 22 males (59.5%) and 15 females (40.5%). The mean age was 47.1 years (range, 30–66 years). The mean final follow-up was 75.1 months (range, 62.5–92.2). Single-level replacement was performed in 27 patients, while 2-level replacement was performed in 10 patients, totaling 47 replaced levels. The most frequently replaced levels were C5–6 in 17 patients and C6–7 in 20 patients (Table 1).
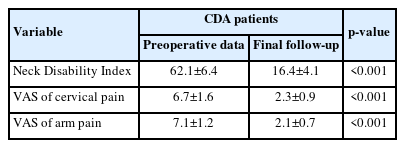
A statistically significant improvement was observed at the end of follow-up in NDI, VAS neck, and VAS radicular (p<0.001) (Table 2).
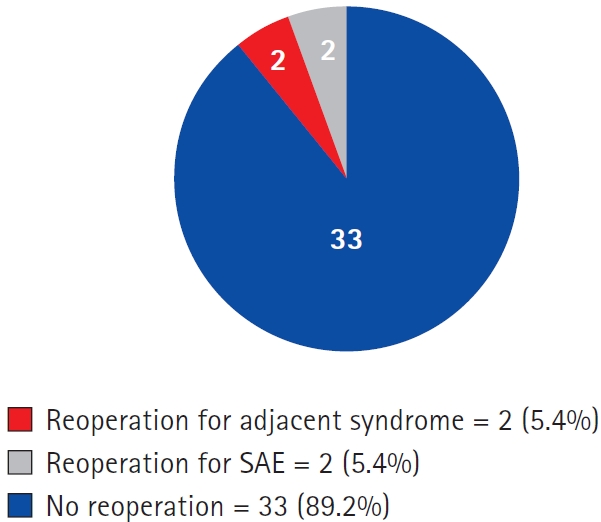
The mean mobility of the operated segment at final follow-up was 8.1°±5.91°, and for the cervical spine, it was 44.8°±9.4°, no statistically significant different was observed at the end of follow-up (Figure 3, Table 3). During follow-up, 4 patients required reintervention, including 2 for adjacent segment syndrome, 1 for possible implant failure, and 1 for a prevertebral collection. Additionally, radiologically, 2 patient (5.4%) exhibited HO, and 5 patients had asymptomatic adjacent segment syndrome (Figure 4, Table 1).

Flexion and extension x-ray examinations demonstrating the mobility of the cervical disc arthroplasty. (A) Extension of C6-C7 cervical disc arthroplasty. (B) Flexion of C6-C7 cervical disc arthroplasty. (C) Extension of C5-C7 cervical disc arthroplasties. (D) Flexion of C5-C7 cervical disc arthroplasties.
The overall CCS at final follow-up was 81.1%. Regarding patient satisfaction, 89.2% of patients reported being satisfied or very satisfied (Figure 5).
DISCUSSION
The novel design of second-generation cervical arthroplasties contrasts with classic ball-and-socket and constrained-fit devices, which theoretically have better-known wear characteristics on their articulating surfaces and allow for little more movement than mere uniaxial rotations [22-24,26]. Our main findings were that, with a minimum follow-up of 5 years, patients undergoing second-generation cervical arthroplasties maintained significant improvement in cervical functional scales and pain compared to their preoperative state. Additionally, they retained mobility at the operated segment and exhibited a low percentage of HO and reintervention due to implant failure or adjacent segment syndrome.
By the end of the follow-up period, 81.1%. had a CCS. These findings, along with prior research on this cervical arthroplasty model and similar ones, suggest that the clinical benefits of disc arthroplasty remain consistent or improve over time [22,27-33]. Phillips et al. [22] reported a 60-month follow-up for CCS outcomes with the M6-C, defining CCS as the absence of SSI at the index level (including revision, removal, reoperation, or supplemental fixation) and the absence of SAE related to the device or index level procedure. CCS also required an improvement of at least 15 points in NDI scores and maintenance or improvement in neurological function, with an overall CCS rate of 70.6%. Coric et al. [31] and Hisey et al. [33] reported 5-year CCS outcomes for the Mobi-C (Zimmer Biomet, Warsaw, IN, USA) and Kine-flex-C (SpinalMotion, Mountain View, CA, USA) CDA devices, respectively [31,33]. Hisey et al. [33] observed that 61.9% of TDR subjects achieved CCS, while Coric et al. [31] reported a CCS rate of 77.2%. Despite variations in CCS definitions, these studies and the present one show favorable CCS outcome over a 5-year period.
Regarding the cervical disability index, neck pain, and radicular pain, a remarkable improvement was observed at the final follow-up, showing statistically significant enhancements. MacDowall et al. [34] reported improvements in their CA group using the Discover artificial disc (DePuy Spine, Johnson & Johnson, New Brunswick, NJ, USA), with NDI scores decreasing from 64 (95% confidence interval [CI], 60.4–67.6) to 36 (95% CI, 31.2–40.8) at 5 years. Additionally, VAS neck scores decreased from 56.9 (95% CI, 50.9–62.9) to 29.1 (95% CI, 23.3–34.8), and VAS arm scores decreased from 57.0 (95% CI, 50.9–63.1) to 24.0 (95% CI, 18.5–29.6). Goedmakers et al. [29] utilized the ActivC flat artificial cervical disc (Aesculap AG, Tuttlingen, Germany) and noted a substantial decrease in NDI scores and VAS scores for neck and arm pain. NDI decreased from 47±17 points at baseline to mean values ranging between 15±14 points at 5 years postsurgery. VAS neck scores decreased from 50±27 points to 17±25 points, and VAS arm scores decreased from 60±24 points to 14±21 points. This improvement has been observed in various studies, including meta-analyses comparing CDA and ACDF, which found that both techniques enhance pain management and improve quality of life [18,19,26,27].
Regarding patient satisfaction, 89.2% of patients reported being satisfied or very satisfied. Hisey et al. [35] found overall patient satisfaction and recommendation rates were 92.0% among CDA patients at 60-month follow-up, and Phillips et al. [36] reported that 88.8% of the CDA patients responded either “very” or “moderately” satisfied with their 5-year outcomes and 94.4% would “definitely” or “probably” recommend the treatment with CDA. These results, in conjunction with the consistent or even enhanced clinical benefits observed over extended follow-up durations for CDA, suggest a positive trend in its long-term effectiveness.
In our study, 10.8% patients required reintervention, 4.4% for adjacent segment syndrome, and 4.4% for SAE adjudicated as definitely device or index level procedure-related (one possible implant failure, and one for a prevertebral collection). Long-term safety outcomes than included reintervention were also comparable for this CDA model and other similar CDA models [22,27,28,36]. Phillips et al. [22] revealed a 5-year rate of reintervention at the index level, including revision, removal, reoperation, or supplemental fixation, of 3.1% in CA subjects implanted with the M6-C, and 8.1% in CDA subjects implanted with the PCM (NuVasive, San Diego, CA, USA) artificial disc [36]. Additionally, Coric et al. [31] found 5-year reintervention rates at the index level of 8.0% in the Kineflex-C CDA group. Phillips et al. [22,36] reported that, at the 5-year follow-up with the M6-C, only 2 of 113 (1.8%) M6-C subjects required SSI due to ASD, with no patients implanted with the PCM requiring SSI for ASD. Furthermore, the 5-year safety outcomes of the ProDisc-C (DePuy Synthes, Raynham, MA, USA) study indicated a 2.9% (3 of 103) rate of SSI, 2 of which were for ASD [37]. Hisey et al. [33] published the 5-year results of the Mobi-C study, which also reported a 4.9% rate of SSI following CDA, 1.8% of which were for ASD. While the rates of reintervention for SAE or ASD have differed across the long-term CDA studies, they have all remained within an acceptable range outcome. Additionally, no significant differences in adverse events were found in some studies comparing CDA and ACDF [27,38]. Despite reoperation rates being significantly lower in the CDA group compared to the ACDF group in most studies, some meta-analyses did not find these differences to be statistically significant [18,26,37].
A significantly higher motion rate in the CDA studies have been found during follow-up compare to ACDF [27,28]. In our study, we found at final follow-up a mean mobility of the operated segment of 8.1°. Phillips et al. [22,36] found at 5 years, the mean flexion/extension ROM in the PCM group was 5.2°±3.8° (range, 0°–16.1°), and at 60 months, the M6-C group-maintained ROM in the index level of 8.33°±4.95°. Younus found that 100% of the subjects in their study preserved motion at the two-year endpoint following CDA [39]. Hisey et al. [35] found that segmental ROM at the treated level was maintained in the CDA group throughout 60-month follow-up. Radiologically, only 5.4% of patient exhibited HO grade 4. Previous studies have shown a variable rate of HO formation with unclear clinical relevance [29,35,36]. Hisey et al. [35] reported the rate of grade 4 HO at the index level at 60-month follow-up was 8.5%. Phillips et al. [36] found 6% (9 of 149) of grade IV HO with bony ankylosis in PCM group. Goedmakers et al. [29] found in ActivC flat artificial cervical disc a 25% of grade 4 HO (spontaneous fusion). Despite the heterogeny of the results of ROM and HO these results show that ROM is maintained during the follow-up.
It is important to acknowledge that the retrospective design of our study inherently imposes limitations on data collection, potentially introducing biases and restricting the ability to establish causal relationships. Furthermore, being a single-center study, our findings may not fully capture the diversity of patient populations and surgical practices seen across different healthcare settings, thus limiting the generalizability of our results. Additionally, while the minimum follow-up period of 5 years provides valuable insights into medium-term outcomes, longer-term follow-up would be beneficial to assess the durability and sustainability of the observed benefits. Moreover, the selection criteria applied, such as age restrictions and exclusion of certain patient groups, may introduce selection bias and restrict the applicability of our findings to broader patient populations with symptomatic cervical disc degeneration. Finally, the focus on a specific type of second-generation cervical disc prosthesis may limit the extrapolation of our results to other implant designs, necessitating caution in generalizing our findings to broader clinical contexts. Strengthening our study, it's worth noting that it is a 5-year series, published without funding from the manufacturer of this arthroplasty, unlike the only other study published at 5 years for this arthroplasty [22].
CONCLUSION
In conclusion, our study highlights the promising outcomes associated with second-generation cervical arthroplasties in the management of symptomatic cervical disc degeneration. Despite its limitations, including sample size and follow-up duration, our findings suggest that patients undergoing these procedures experience sustained improvements in cervical functional scales and pain relief over a 5-year period. Additionally, the preservation of segmental mobility and low incidence of complications such as HO and reintervention underscore the potential benefits of second-generation cervical arthroplasties. Future research endeavors, including larger prospective studies with longer follow-up periods, are warranted to further elucidate the efficacy and long-term outcomes of these surgical interventions.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.