Lateral Approach With and Without Posterior Stabilization for Lumbar Pyogenic Spondylodiscitis Treatment: A Retrospective Study With an Average Follow-up of 7 Years
Article information
Abstract
Objective
The aim of this study was to present the results after an average of 7-year follow-up in patients diagnosed with pyogenic spondylodiscitis (PS) treated with lumbar discectomy and fusion through minimally invasive lateral lumbar interbody fusion (LLIF), with and without posterior stabilization.
Methods
In this retrospective analysis, patients diagnosed with PS treated with minimally invasive LLIF were studied. Variables included C-reactive protein (CRP), blood cultures, and findings on magnetic resonance imaging, computed tomography, and x-ray examinations. The classification of PS according to Pola was recorded.
Results
Twenty-one patients were male (70%) and 9 were female (30%), with an average age of 67.6 years (range, 33–85 years). The average follow-up was 83.2 months (range, 12–160 months). In 7 out of the 30 cases, treatment consisted exclusively of discectomy and anterior fusion through LLIF. Bone consolidation was observed in 27 out of 30 cases (90%), with an average postoperative CRP level of 5.77±5.00 mg/L at the end of follow-up.
Conclusion
The treatment of lumbar PS through debridement and minimally invasive LLIF, with or without posterior stabilization, resulted in infection control over an average follow-up of 83 months.
INTRODUCTION
Pyogenic spondylodiscitis (PS) consists of the infection of the vertebral disc unit, usually determined by hematogenous seeding, although it can also occur after spinal interventions. Recent studies show an increase in its incidence of around 140% in the last 2 decades, due to the growing number of chronically debilitated patients and an increase in invasive spinal procedures [1,2].
The diagnosis of PS is often delayed as symptoms are nonspecific, most commonly presenting with lower back pain (85%), followed by fever (48%), and paresis (32%). The peak prevalence occurs between 50–70 years, with a male predominance and a male-to-female ratio of 2:1 [3-5]. The most frequently isolated microorganisms are Staphylococcus aureus, Streptococcus sp, and gram-negative bacilli [3-5].
Medical or conservative treatment is the first choice and consists of antibiotic therapy and immobilization with a rigid brace. The main criterion for continuing conservative treatment is a good response to antibiotics during the first 2 to 3 weeks. Surgical treatment is reserved for cases where conservative treatment fails, evidenced by the progression of bone destruction, spinal instability, and/or neurological deficits [3,4,6-9].
There is still controversy regarding the preferred surgical treatment for PS. Anterior lumbar discectomy and fusion allow adequate debridement of necrotic tissues and reconstruction of the anterior column, providing solid stabilization [7-10]. Among these techniques, minimally invasive lateral lumbar interbody fusion (LLIF) allows for adequate debridement and solid fusions through smaller incisions, with less soft tissue damage, minimal bleeding, shorter hospital stays, less postoperative pain, and faster recovery [7,10-13]. Its indication in spondylodiscitis as the sole means of stabilization or "stand alone" is controversial and has not been widely adopted [4,7,11].
The aim of this study is to describe the clinical and radiological outcomes of a series of patients diagnosed with lumbar PS treated with LLIF, with or without posterior stabilization. The secondary objective is to analyze those patients treated with this technique as the sole approach, without further posterior stabilization, which we will refer to as LLIF "stand alone" (LLIF SA).
MATERIALS AND METHODS
The study was approved by the Research and Ethics Committee of the Italian Hospital of Buenos Aires (protocol number 6134 - PRIISA file 5182) and adheres to the principles set forth in the Helsinki Declaration. Confidentiality of data was maintained in accordance with the National Law 25326 for Personal Data Protection.
A retrospective analysis was carried out on a cohort of patients diagnosed with PS, treated with lumbar discectomy and fusion through minimally invasive lateral approach (LLIF) with and without posterior stabilization, at a high complexity center, between February 2010 and September 2022, with a minimum follow-up of 12 months.
Data collection was performed from the electronic medical records of our Institution. Patients over 18 years old with clinical and imaging parameters consistent with PS in the lumbar region, and who met certain criteria for surgical intervention, were included. Patients diagnosed with L5–S1 PS, primary or metastatic vertebral tumors, vertebral tuberculosis, and cervical and thoracic spondylodiscitis above the thoracolumbar junction were excluded.
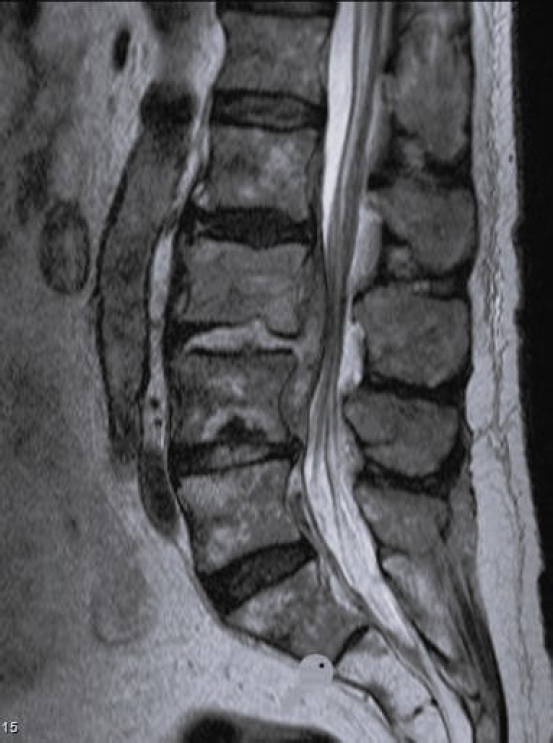
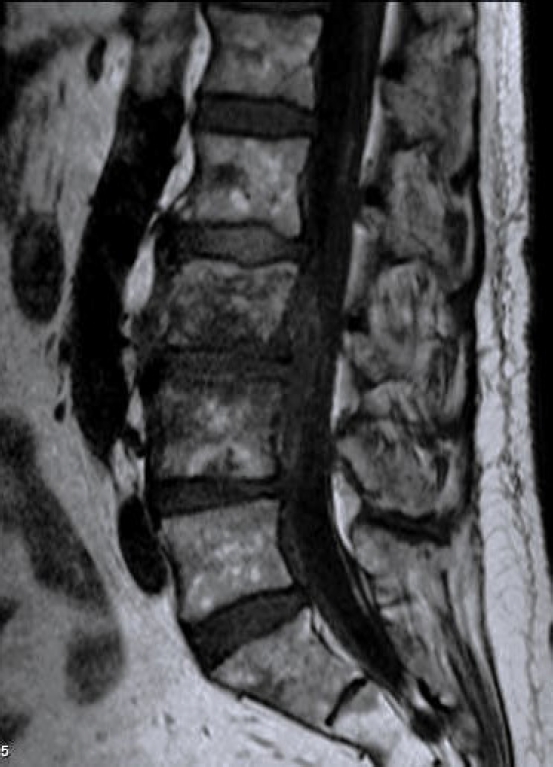
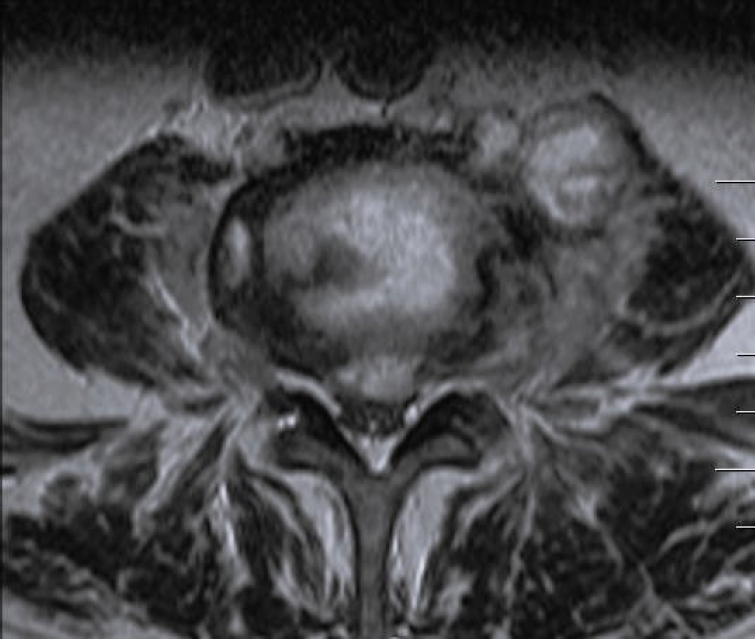
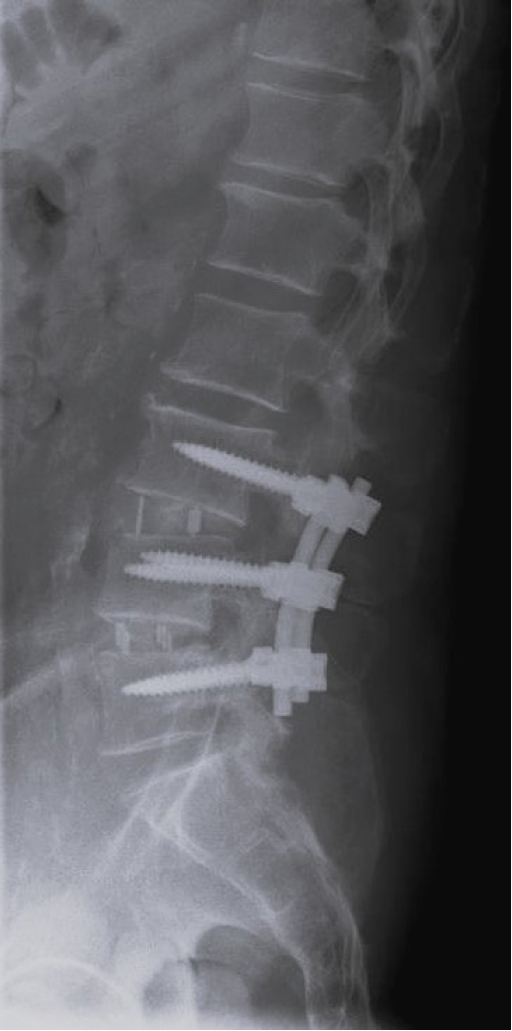
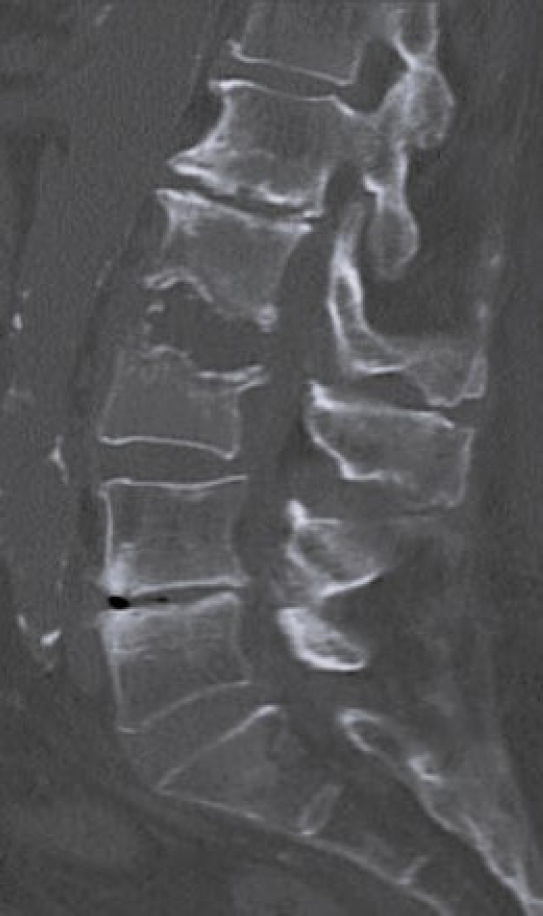
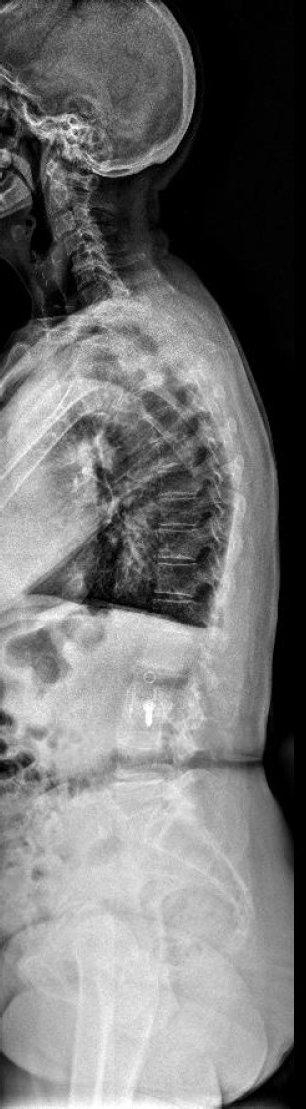
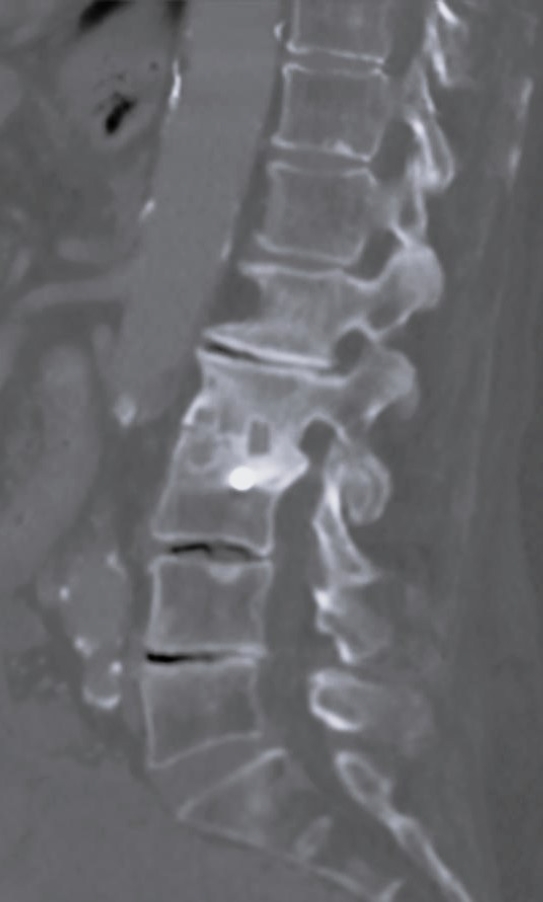
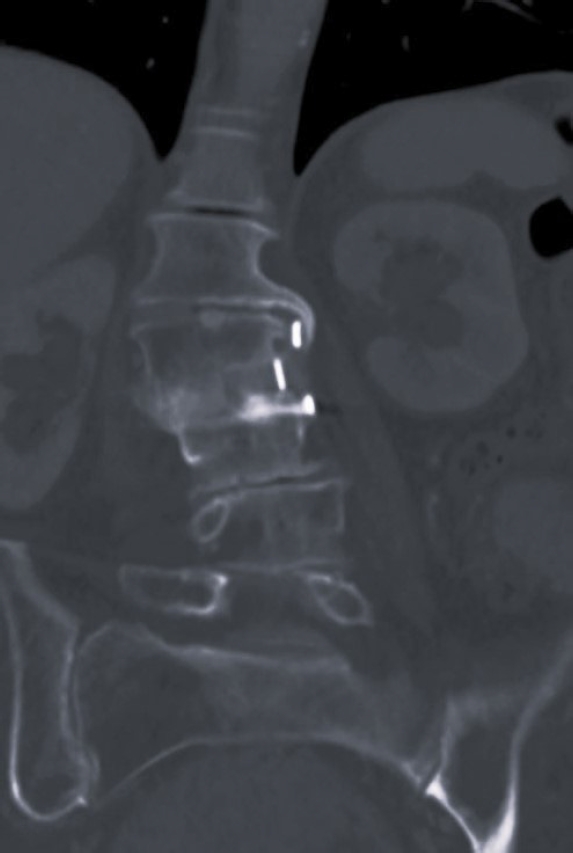
Laboratory tests evaluated in the study included C-reactive protein (CRP) and blood cultures. Imaging analysis included magnetic resonance imaging (MRI), computed tomography (CT), and x-rays. The preoperative MRI was used to classify spondylodiscitis according to the classification of Pola et al. [14] and to establish the distribution of the pathology by disc level (Figures 1–3). Fusion assessment was based on x-rays and/or CT scans, considering mature fusion when trabecular bone bridges were present between the vertebral endplates with graft incorporation at both ends and no radiolucent spaces between the graft and the vertebral endplate (Figures 4–6). The absence of these findings indicated pseudarthrosis, considered as treatment failure, in addition to characteristic mechanical pain and other radiological signs of failure such as loosening of material. When the images were inconclusive for either solid fusion or pseudarthrosis and there were no compatible clinical changes or other signs of failure detected, it was recorded as undetermined [15,16].
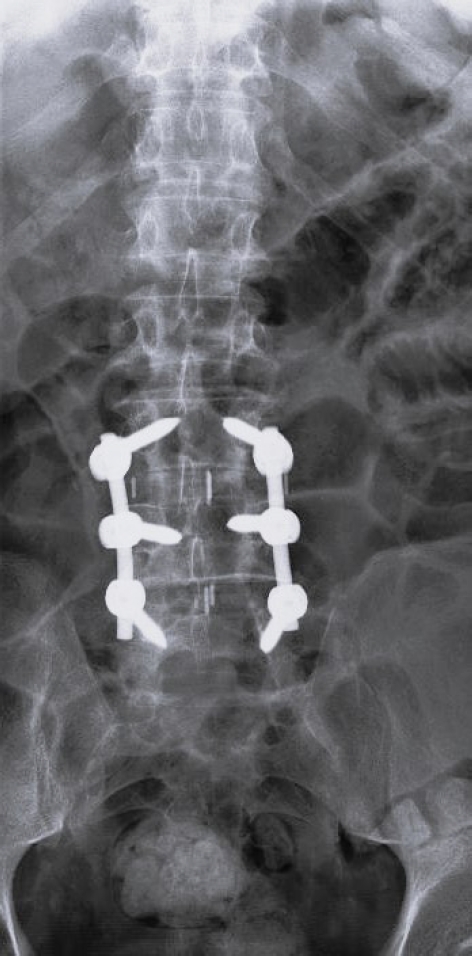
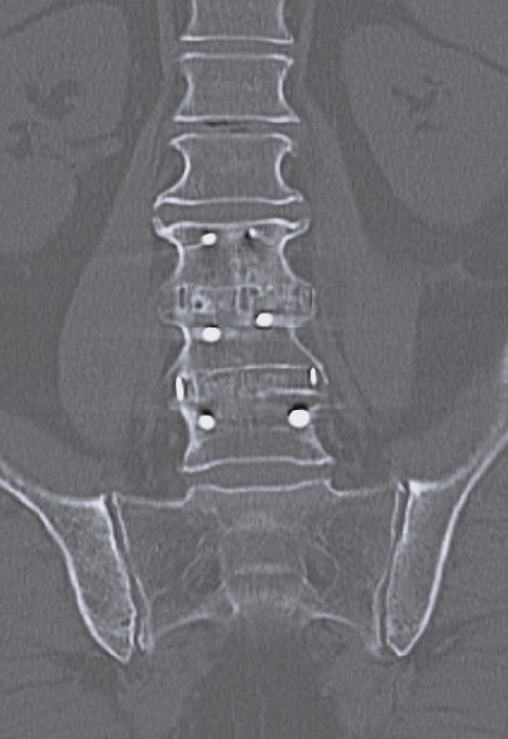
Anterior-posterior x-ray at 1-year postoperation after lateral lumbar interbody fusion and posterior stabilization.
Statistical analysis was performed using Stata 11 (StataCorp LLC, College Station, TX, USA). Continuous variables were evaluated using a paired t-test. The decrease in CRP levels in cases treated with LLIF SA was specifically studied using a one-sample t-test. A p-value of <0.05 was considered statistically significant for all tests.
1. Medical Treatment
During hospital admission all patients were tested with urine and blood cultures, in those in which no bacteria was identified, disc and vertebral puncture were performed before antibiotic treatment. Before surgical intervention, all patients received medical treatment empirically based on broad-spectrum intravenous antibiotics, adjusted later based on cultures and germ sensitivity. Antibiotic treatment continued postsurgery as per Infectious Diseases recommendations. Patients treated with LLIF SA were also prescribed a rigid brace for 6–8 weeks. Infection control was determined by normalization of CRP values and achieving mature fusion on x-rays and/or CT scans.
2. Surgical Technique
General anesthesia was administered in all cases. The patient was positioned on a radiolucent surgical table with flexion capacity near its midsection. A strict lateral decubitus was preferred, on the right side, with the greater trochanter at the breakpoint of the table, and positioning was completed using fluoroscopy to obtain strict anterior and lateral images at the target disc. Neurophysiological monitoring was performed with somatosensory evoked potentials and electromyography. Depending on the disc level to be treated, a lumbotomy or thoraco-lumbotomy was performed, with a 3- to 4-cm oblique incision according to premarking. Rib resection was performed when necessary at certain levels, completing the approach with blunt dissection. A minimally invasive LLIF retractor was used in all cases. After completing the approach and inserting the retractor, discectomy was performed, infected tissue was debrided, and the residual space was structurally reconstructed with a cage (polyetheretherketone [PEEK] or titanium) or tricortical iliac crest graft, depending on the observed bone defect. Pedicle screws were placed open or percutaneously in cases requiring posterior stabilization.
RESULTS
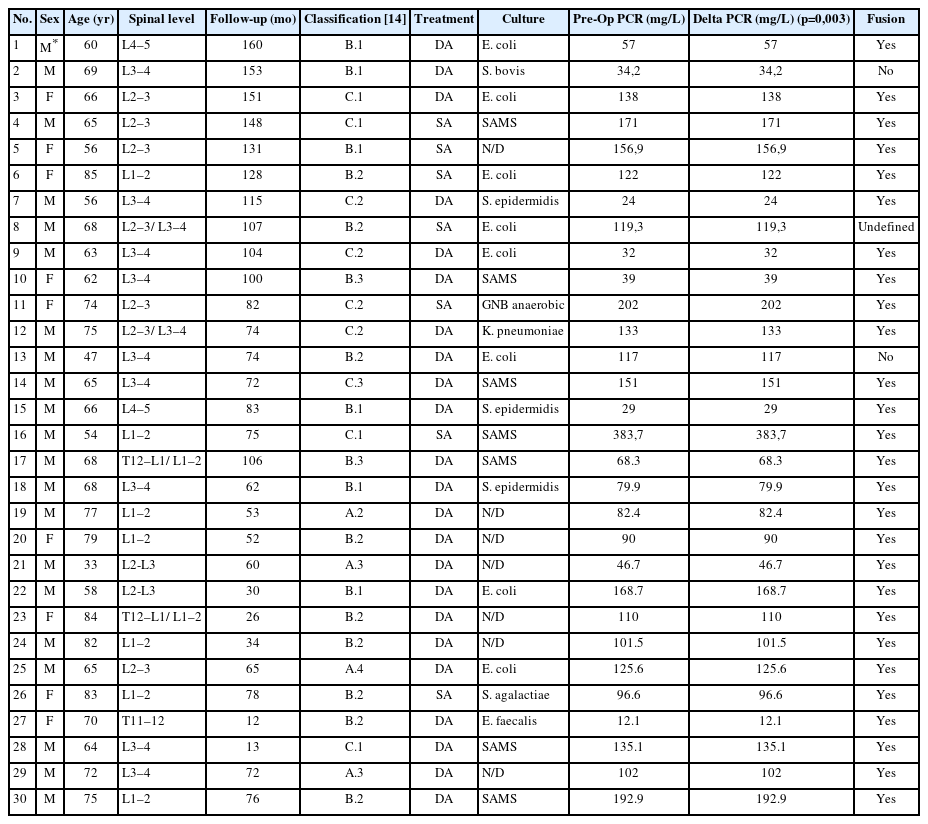
Out of a total of 107 vertebral infections treated between February 2010 and September 2022, 43 patients were identified who were treated with LLIF, with or without posterior stabilization. Three patients were excluded due to tuberculous infection, 8 were lost to follow-up, and 2 died—one from bacterial endocarditis and the other from sepsis. A total of 30 patients met the inclusion criteria. The demographic characteristics of these patients are presented in Table 1.
Twenty-one were male (70%). Age ranges from 33 to 85 years (mean, 67.6 years). Follow-up ranges from 12 to 160 months (mean, 83.2 months). In 7 out of 30 cases (23%), treatment consisted exclusively of discectomy and anterior fusion through LLIF (Table 2). Of the 23 cases treated with combined anterior-posterior, 14 underwent posterior instrumentation and fusion (47%), and 9 received posterior stabilization with percutaneous screws (30%).
Our cohort analysis based on the classification of Pola et al. [14] showed that half of the cases were distributed between B.2 (30%) and B.1 (20%), representing lesions with significant bone destruction and/or mechanical instability but without neurological compromise or epidural abscess. Of the 7 patients treated with exclusive LLIF approach, 3 were classified as B.2, 2 cases as C.1, and the remaining 2 as B.1 and C.2 (Figures 7–9).
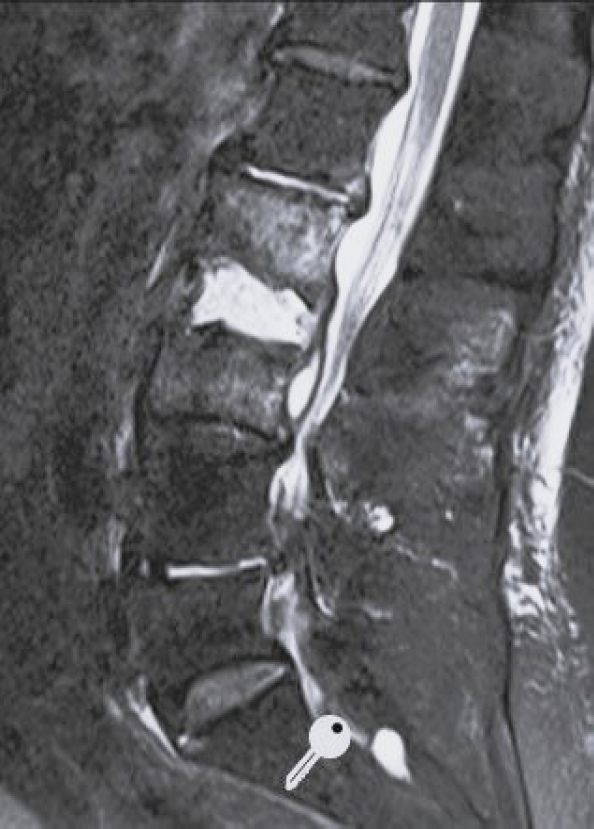
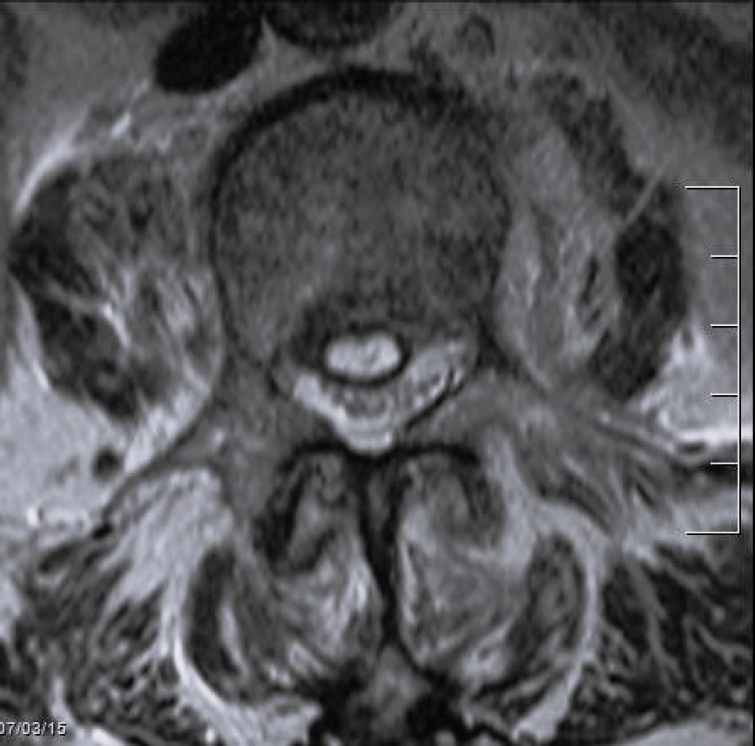
Short tau inversion recovery sagittal magnetic resonance imaging of a 83-year-old female. Spondylodiscitis L2–3 with epidural abscess.
Regarding the distribution of affected levels, 5 patients had 2 affected levels, making a total of 35 discs treated for pyogenic infection. The most commonly affected level was L3–4 with 12 cases, followed by L1–2 and L2–3 each with 9 cases. The remaining 5 cases were distributed among T11–12 (n=1), T12–L1 (n=2), and L4–5 (n=2).
In 18 out of 30 cases (60%), preoperative bacteriological rescue was achieved, either through blood cultures or biopsy, with Escherichia coli being the most frequently isolated germ (23%), followed by methicillin-resistant Staphylococcus aureus (MRSA) (13%). Of the 12 negative cases at this stage, 6 remained negative in surgical biopsies, while in the other 6, MRSA was isolated in 3 cases, E. coli in 2, and K. pneumoniae in the remaining case. When combining pre and postoperative cultures, E. coli was the most common germ isolated, followed by S. aureus, and negative cultures.
The preoperative CRP mean was 110.97 mg/L, while the postoperative CRP mean was 5.77 mg/L at the end of follow-up. A t-test showed a significant effect of the LLIF debridement and fusion on CRP, with a mean decrease of 105.2 mg/L (95% confidence interval [CI], 77.75–132.64; p<0.0001) by the end of the follow-up. Specifically analyzing patients treated with LLIF SA, the mean decrease in CRP at the end of follow-up was 175.14 mg/L (95% CI, 85.67–264.61), which was also statistically significant (p=0.003).
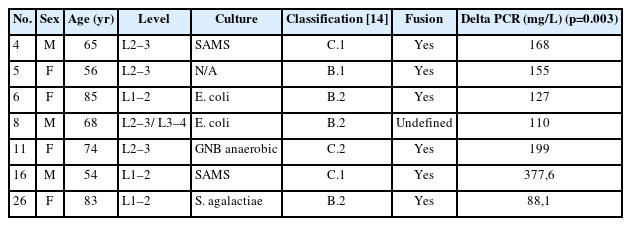
Bone consolidation was evidenced in 27 out of 30 cases (90%), with pseudoarthrosis in 2 cases and 1 case being indeterminate for fusion or pseudoarthrosis, but with no symptoms. Within the group of patients treated with LLIF SA, 6 out of 7 showed mature fusion (Figures 10–12), while the remaining case had an undetermined bone consolidation after 9 years of follow-up but remained asymptomatic. This particular patient had 2-level spondylodiscitis (L2–3 and L3–4), with L2–3 classified as B.2 and L3–4 classified as A.2. Due to the patient's frail clinical condition, with hepatic encephalopathy and overall poor health, LLIF SA was performed.
DISCUSSION
Currently, there are no standardized and consensus therapeutic recommendations establishing strict guidelines for the surgical treatment of lumbar PS [14]. Available evidence consists of retrospective analyses based on small populations [17-19]. Without prospective randomized studies, the level of evidence for therapeutic guidelines will remain low. Additionally, the heterogeneity in the clinical-radiographic presentation of this pathology increases the controversy regarding the most appropriate approach in cases requiring surgical intervention [20].
Since these are potentially severe infections affecting a fragile population, often with delayed diagnosis [3,4,6,20], surgical treatment is challenging, with clear surgery goals: to control the local infection and prevent sepsis, restore or maintain the stability and alignment of the spine, to control pain, prevent and treat neurological complications, establishing an environment that promotes arthrodesis to reduce recurrence. Literature supports the use of anterior and posterior approaches, as well as a combination of both [10,21]. Reports of cases treated using the LLIF SA approach are scarce. Unlike the formal anterior approach, LLIF does not mobilize major vessels, leading to reduced surgical time, blood loss, and postoperative pain [3,4,10,11,18,20,22]. Another important advantage is that in obese patients, who have higher rates of infectious complications with posterior approaches, lateral decubitus is better tolerated and reduces the working distance to reach the disc space [13,15].
On the other hand, the lateral approach allows complete debridement under direct vision, avoiding bacterial spread to the posterior paravertebral space and instrumentation [18,22]. However, the fixation rigidity is not as consistent as with posterior instrumentation. Given these conditions, the combination of anterior and posterior approaches is widely used [18,22,23].
In line with the literature reporting a male-to-female ratio of 2:1 and a peak prevalence between 50–70 years [3,14], the gender distribution in our series was 70% male, with a mean age of 67.6 years. The preoperative bacterial isolation rate was 60%, similar to previous studies. However, the most frequently isolated germ in our series was E. coli, unlike previous reports where S. aureus predominated [3,4,6,8,9,13]. This difference could be due to a higher incidence of urinary infections as the primary focus in our population.
Studies focusing on the treatment of PS solely through lateral approach are limited. Patel et al. [13] reported 6 cases with infection control and stable fusion in 5, while 1 case required posterior stabilization after 2 months due to implant loosening. The inclusion criteria for this series included instability, intractable pain, neurological deficits, and failure of conservative treatment. In contrast, patients with instability and neurological deficits in our cohort were additionally treated with posterior decompression and stabilization as the first step. Similarly, Timothy et al. [21] reported 11 patients treated with LLIF stand alone, following criteria similar to ours, achieving solid fusion in all cases without infection recurrence and without requiring surgical revision.
When analyzing LLIF stand-alone cases according to the classification of Pola et al. [14], we did not observe a direct relationship between the classification and the chosen approach. This single lateral approach was selected due to the patients' clinical instability, aiming to avoid an additional surgical procedure, reducing surgical time and the consequent prolonged anesthesia. The common factor among these cases was the poor general health at the time of surgery, neurological integrity, and absence of severe local kyphosis. We believe this is essential, as the technique was not forced, and we are aware that in the presence of mechanical instability and/or neurological compromise, the stand-alone approach may be insufficient for stability and/or neurological decompression.
This study has several limitations. Firstly, it's a retrospective study with data collected from our hospital’s medical records, which could introduce a selection bias. To mitigate this bias, strict inclusion and exclusion criteria were applied to homogenize the sample. Secondly, although the number of cases in the series (30) and the average follow-up period (83 months; range, 12–160) are not large, they are consistent with most reports [18,19]. In fact, this series has the longest follow-up period to our knowledge. A third weakness of our study, inherent to the pathology evaluated, is the sample's heterogeneity. This is due to the diversity of germs capable of causing PS, their variable virulence, the variability in patients' frailty and clinical instability, and the diversity of options at the time of surgical resolution (autologous cancellous bone vs. tricortical graft, PEEK cages vs. titanium, percutaneous fixation, or open fusion through a posterior approach, among others). Unfortunately, the limited case numbers of this pathology did not allow us to create patient subgroups based on these variables. Lastly, the evaluation of patient progress using polymerase chain reaction was not based on a predetermined protocol. This information was collected at different stages of case evolution; hence only values at the time of diagnosis (pretreatment) and at the end of follow-up treatment were considered.
CONCLUSION
The treatment of lumbar PS through debridement and minimally invasive lateral transpsoas fusion, with or without posterior stabilization, resulted in infection control with a mean follow-up of 83 months. However, prospective, randomized, and likely multicenter studies are needed to obtain a more representative sample of the population and to precisely establish when to indicate a combined anterior/posterior approach and when to perform an LLIF stand-alone procedure.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.