Validation of a Novel Provocative Examination Maneuver for the Diagnosis of Lumbar Foraminal Stenosis Through Selective Nerve Root Block Outcomes
Article information
Abstract
Objective
Lumbar foraminal stenosis can lead to nerve impingement and radiculopathy in a subset of patients. Imaging findings have a high false-positive rate; therefore, physical examination findings are of critical importance prior to interventional procedures. Kemp test is a provocative maneuver that has traditionally been used for the diagnosis of facet joint disease. However, it has shown an increased likelihood of foraminal stenosis diagnosis in small series. To evaluate the diagnostic accuracy of a modified version of Kemp test (the Singh-Hartl foraminal compression test) in the diagnosis of symptomatic foraminal stenosis. The results were validated using selective nerve root block (SNRB) patient-reported outcomes.
Methods
Fifty consecutive patients referred for SNRB on the basis of a history of radiculopathy, imaging findings, and physical examination were included. The participants were asked to perform lumbar extension followed by ipsilateral rotation to the painful side. The test was positive if there was reproduction of radicular symptoms down the ipsilateral lower extremity within 10 seconds of sustained positioning. Patients then underwent SNRB to the suspected level, reporting visual analogue scores (VAS) (range, 1–10) of ipsilaterally injected leg pain before and after SNRB immediately before and at 15-minute postprocedure. VAS improvement was calculated as a percentage, with a foraminal stenosis diagnosis confirmed when 75% calculated pain relief was achieved by injection. The results of the Singh-Hartl foraminal compression test were then compared with these outcomes to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) considering 2 different cutoffs (at least 75% or 80% improvement in VAS score). Additionally, 95% rectangular confidence intervals (CIs) were calculated.
Results
Twenty-eight participants (56%) were women, with an average age of 47.3 years. L4 was the most common nerve root involved (n=20). Using 75% improvement in the VAS as the cutoff, the Singh-Hartl foraminal compression test had a sensitivity of 0.72 (95% CI, 0.53–0.87), specificity of 0.57 (95% CI, 0.34–0.78), PPV of 0.70 (95% CI, 0.56–0.79), and NPV of 0.60 (95% CI, 0.43–0.75). When using 80% improvement in VAS score as the cutoff, the test had a sensitivity of 0.64 (95% CI, 0.41–0.83), specificity of 0.43 (95% CI, 0.24–0.63), PPV of 0.47 (95% CI, 0.36–0.58), and NPV of 0.60 (95% CI, 0.43–0.75).
Conclusion
The Singh-Hartl maneuver exhibited moderate to strong ability to rule-in the diagnosis and a moderate ability to rule out the diagnosis of foraminal stenosis when using a cutoff of 75% improvement in radicular symptoms post-SNRB. Studies evaluating the result of this physical examination test in conjunction with severity of imaging findings are warranted.
INTRODUCTION
Low back pain (LBP) is a pervasive complaint reported by 80% of Americans at one point during adulthood [1]. The incidence of LBP is greatest during the 5th and 6th decades of life, and there is a 13.1% point prevalence in adults ages 20 to 69 [2]. Eighteen point nine percent of patients with LBP also have lumbar spinal stenosis (LSS) [3], compared with a rate of LSS in the general population of 8%–11% [4]. LSS is most commonly a degenerative process and can occur in any region of the spinal canal or foramina [5]. When LSS occurs centrally, there is compression of the thecal sac and nerve roots leading to neurogenic claudication. Foraminal stenosis is usually due to chronic degenerative changes involving the intervertebral disc, facet joints, or osteophyte formation [6]. These stenotic changes affect both the spinal nerve and the vasculature as they exit the foramen, and can result in radiculopathy [7]. Foraminal stenosis is a major cause of neuropathic pain [8], and many patients with foraminal stenosis report some degree of radicular pain [9-12], compared with 3%–5% of the general population [13].
Patients with lumbar radiculopathy can present with pain in their gluteal region and lower extremities, in addition to weakness and paresthesia in their lower extremities depending on the nerve root involved [9-11]. Patient’s radiculopathy symptoms may be unilateral or bilateral, with one study reporting a greater incidence of unilateral symptoms [9,10]. Multiple studies have assessed the distribution of symptoms in patients with LSS, and have found that the most common symptom is LBP (95%–100% of patients), leg pain (71%), lower extremity paresthesia (45%), and weakness (31%–33%) [9,10]. Seventy percent of patients report pain in both their low back and extremities, while 25% only report leg pain [9]. Radiculopathy symptoms tend to be aggravated by exercise, standing, and walking, and alleviated by sitting [9,10]. Patients may also present with kyphosis [10].
The diagnosis of lumbar foraminal stenosis requires a multifaceted approach involving the patient’s history, physical exam, and imaging studies [7,9,10,14,15]. Magnetic resonance imaging (MRI) and computed tomography (CT) scans are used to assess and measure neural compression; however, they can present conflicting results for the diagnosis of foraminal stenosis and radiculopathy [9]. Foraminal stenosis on imaging does not guarantee the presence of pain, but rather indicates presence of the degenerative process, and increased risk of developing radiculopathy [7]. In addition, studies have found that when imaging is used to diagnose foraminal stenosis, there is a 30%–40% false-positive rate [16]. In another study of 98 asymptomatic patients, with an average age of 42.3 years old, 7% had radiographic lumbar foraminal stenosis [1]. Thus, physical examination is crucial in determining a diagnosis, with imaging useful in assessing the degree and etiology of stenosis [7].
Selective nerve root block (SNRB) can be used to confirm the presence of foraminal stenosis. In a systematic review, Datta et al. [17] found support for their use as a diagnostic and therapeutic option for radicular pain, and found that subsequent surgical intervention confirmed the accuracy of the diagnostic SNRB in identifying the level of radicular pain. Similar findings have been reported by others, with accuracy rates ranging from 87%–93% [18,19]. Furthermore, in a prospective controlled study of patients with radiculopathy, investigators found that diagnostic SNRB had an accuracy, positive predictive value (PPV), and negative predictive value (NPV) of 73%, 77%, and 71%, respectively in achieving clinically significant (>70%) pain relief [20].
Despite this data from an interventional procedure, there is currently no definitive clinical finding for lumbar foraminal stenosis [14]. Instead, physicians consider the entire spectrum of a patient’s symptoms, physical exam findings, and imaging when formulating a diagnosis. The Kemp test, which is also called the Extension-Rotation test or Quadrant test, is a provocative test used for evaluating facet joint pain [15]. However, studies have also demonstrated that when the Kemp test is positive, it also increases the likelihood of a foraminal stenosis diagnosis [10,15].
This lack of a definitive clinical exam to identify lumbar foraminal stenosis presents difficulties for making an accurate diagnosis, especially in ambiguous situations when radicular or sensorimotor symptoms do match with specific nerve root distributions. This study aims to validate the diagnostic utility of a novel provocative test, the Singh-Hartl foraminal compression test, in evaluating lumbar foraminal stenosis. The Singh-Hartl foraminal compression test represents a modification of the Kemp test, and involves the patient maintaining a sustained extension and rotation to reproduce their radicular leg pain.
MATERIALS AND METHODS
A prospective trial examining the diagnostic validity of the proposed Singh-Hartl foraminal compression test to diagnose foraminal stenosis was initiated after obtaining approval from Weill Cornell Institutional Review Board. Subjects were recruited from a university medical center multispecialty spine clinic between January and December 2019 when they were scheduled for SNRB for radicular pain due to foraminal stenosis. Subjects were referred for injection if they had typical symptoms, physical examination findings, and radiographic data suggesting that foraminal stenosis was present, and failure of conservative treatment of oral medications and physical therapy. Inclusion criteria included age greater than 18 years old and referral for injection after presenting with lower back pain symptoms thought to be related to foraminal stenosis. Subjects reported their baseline pain severity by indicating their pain level on a 10‐point visual analogue scale (VAS). All patients provided informed consent after discussion of risks, benefits, and alternative for any interventional or surgical procedures as well as participation in the study.
The Singh-Hartl maneuver was administered by physicians who specialized in physical medicine and rehabilitation and interventional pain techniques, using the following technique: The subject was asked to perform lumbar extension followed by ipsilateral rotation to the painful side (Figure 1). The test was determined to be positive if there was reproduction of the patient’s pain with radicular symptoms down the ipsilateral lower extremity if symptoms were elicited between 0 and 10 seconds of sustained positioning.

The Singh-Hartl foraminal compression test. (A) The subject in neutral position. The examiner places one hand on the lumbar spine for support. Posterior (B) and lateral view (C) of the patient being pulled into lumbar extension. (D) For assessment of left-sided foraminal stenosis, the patient is slightly rotated and flexed to the left side, and remains in that position for 10 seconds. A positive test is signified by the reproduction of left-sided radicular pain down the leg.
Results of the test were documented and patients subsequently underwent SNRB. With the use of sterile technique, 10-mg dexamethasone, 3 mL of 1% lidocaine were injected into under fluoroscopic guidance. Injection of contrast material (Omnipaque, GE Healthcare, Princeton, NJ, USA) confirmed correct needle placement near the target nerve root exiting the interverbal foramen before administration of injectate. Subjects then rated their pain on a 10‐point VAS identical to the one used before the procedure 10 to 15 minutes postinjection. The percentage of pain relief following injection was calculated as the difference between preprocedure VAS score and postprocedure VAS, divided by the preprocedure VAS. Foraminal stenosis was assumed to be the pain generator when 75% calculated pain relief was achieved by injection. This was also evaluated using a more stringent criterion of 80% pain relief by VAS to increase confidence in excluding false positives.
Sensitivity, specificity, PPV, and NPV were calculated considering 2 different cutoffs (at least 75% or 80% improvement in VAS score) for the modified Kemp maneuver. 95% rectangular confidence intervals (CIs) were then calculated for the modified Kemps maneuver.
RESULTS
A total of 50 subjects were enrolled in this study of whom 28 (56%) were female. The average age of the study participants was 47.3±11.4 years old. Demographic subject information can be found in Table 1. Pain scores before and after injection as well as percentage of estimated pain relief are summarized in Table 2. Of the patients who experienced at least 80% improvement of VAS score (n=22), there were 6 cases of pathology of L2 nerve root, 2 at L3 nerve root, 11 at L4 nerve root, and 3 at L5 nerve root. Of the patients who experienced at least 75% improvement of VAS score (n=29), there were 7 cases of pathology of L2 nerve root, 3 at L3 nerve root, 13 at L4 nerve root, and 4 at L5 nerve root.
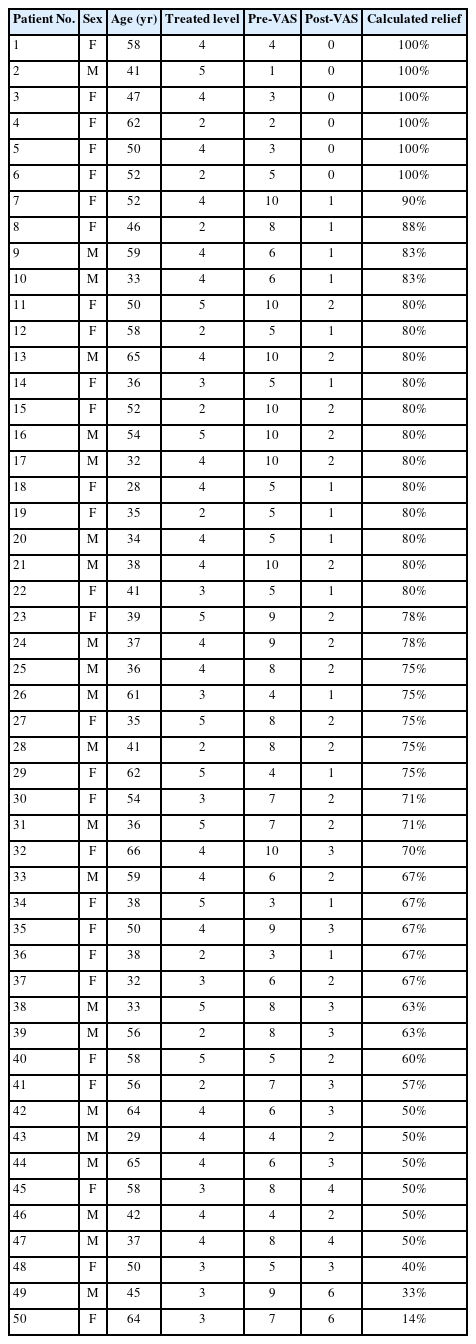
Visual analogue scale (VAS) pain scores before and after selective nerve root block and percentage of relief
For results of sensitivities and specificities, see Table 3. Using 75% improvement in VAS as the cutoff, the foraminal compression test had a sensitivity of 0.72 (95% CI, 0.53–0.87) and specificity of 0.57 (95% CI, 0.34–0.78). When using 80% improvement in VAS score as the cutoff, the foraminal compression test had a sensitivity of 0.64 (95% CI, 0.41–0.83) and specificity of 0.43 (95% CI, 0.24–0.63). A summary of PPV and NPV is outlined in Table 4. When using 75% relief as the threshold for confirming pain generator, the Singh-Hartl foraminal compression test had a PPV of 0.70 (95% CI, 0.56–0.79) and a NPV of 0.60 (95% CI, 0.43–0.75). PPV decreased to 0.47 (95% CI, 0.36–0.58) when using 80% relief as the threshold for confirming foraminal stenosis as the pain generator.

Sensitivity and specificity values using ≥75% and ≥80% perceived relief as confirmation of foraminal stenosis
1. Case Example
A 59-year-old male presented with 6 months of right sided back pain and radiculopathy into the right leg. He has a history of a right L5–S1 microdiscectomy 10 years prior, which at the time resulted in complete resolution of symptoms until this episode. The radicular pain radiates from the low back to lateral aspect of the thigh, down through the front of the leg. He also endorsed a burning sensation of the right foot. These symptoms are distinct from the pain he had years ago with the herniated disc, which was focused more in the buttocks and down the posterior aspect of the leg down to the calf.
On physical examination, decreased sensation to light touch was noted in the lateral aspect of the leg, with hyperesthesia over the dorsum of right foot. His Singh-Hartl foraminal compression test was positive when bending to the right side, and negative when bending to the left. Straight leg raise was also negative bilaterally. MRI of the lumbar spine demonstrated no central canal stenosis and laminar defect at the L5–S1 level. There was severe L5–S1 disc degermation with collapse and mild retrolisthesis of L5 relative to S1. There was moderate foraminal stenosis on the left side, but severe foraminal stenosis on the right causing compression of the exiting L5 nerve root (Figure 2A-C).
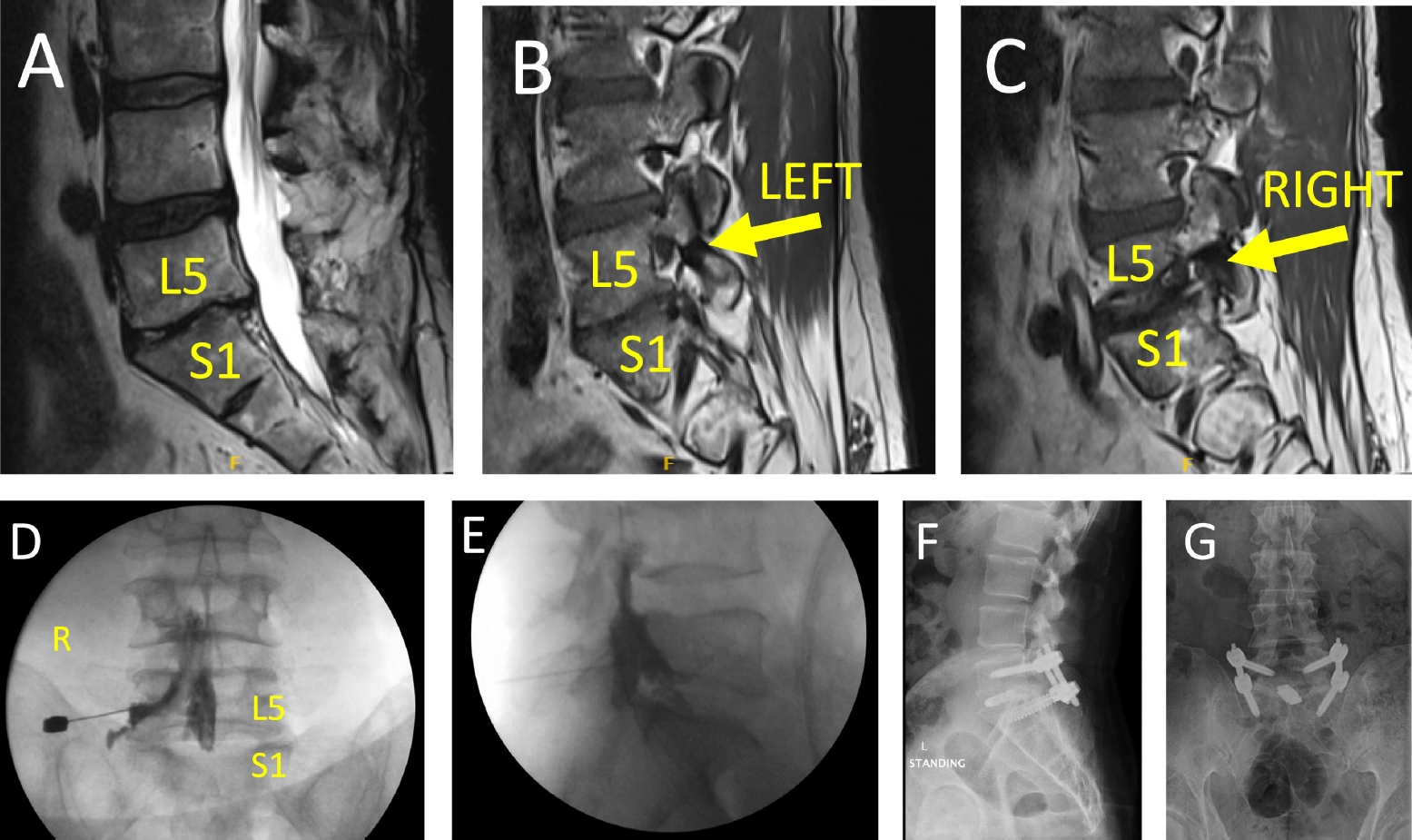
Case example demonstrating change in management based on Singh-Hartl foraminal compression test results. (A) T2-weighted sagittal magnetic resonance imaging (MRI) of a 59-year-old patient with right low back pain and right leg radiculopathy. There is no central canal stenosis, but advanced L5–S1 degeneration. (B, C) T1-weighted sagittal MRI of the left and right foramen, respectively, demonstrating moderate stenosis on the left, and severe stenosis on the right with compression of the exiting nerve root. (D, E) After positive Singh-Hartl foraminal compression test on the right, but not on the left during physical examination, a right L5–S1 transforaminal epidural steroid injection was performed. The patient had complete resolution of symptoms for 2 hours after this procedure, prompting surgical consultation. Lateral (F) and anterior-posterior (G) standing x-rays status post L5–S1 transforaminal interbody fusion with left L5–S1 facetectomy and percutaneous pedicle screw instrumentation. The patient had complete resolution of symptoms without complications or reoperation at 6 month-follow-up.
Based on constellation of history, physical exam findings, and imaging, the patients was scheduled for right L5–S1 transforaminal epidural steroid injection (Figure 2D-E). For 3 hours following this procedure, the patient reported complete relief of radiculopathy. With this information, the patient had surgical consultation and ultimately underwent a minimally invasive L5–S1 transforaminal interbody fusion with right L5–S1 facetectomy, and percutaneous pedicle screw instrumentation (Figure 2F-G). The patient had excellent response to the surgery, with complete resolution of symptoms by 6-month follow-up with no complications or reoperations.
DISCUSSION
This study is one of the few studies to evaluate the diagnostic validity of a modified Kemp maneuver to diagnose lumbar foraminal stenosis. The results from this study demonstrate that when considering 75% relief on VAS with a selective nerve root blook, the proposed Singh-Hartl method has a moderately high sensitivity and moderate specificity for detecting lumbar foraminal stenosis. In this scenario, the PPV and NPV are moderately high as well. However, upon utilizing a more stringent criteria of 80% relief on VAS with a SNRB, sensitivity, specificity, and PPV decrease. Overall, sensitivity and specificity are not robust enough to diagnose foraminal stenosis by physical exam only.
There are a few prior studies that have indirectly evaluated for a positive Kemp test and the presence of lumbar stenosis though there is a fair amount of heterogeneity in definitions in these studies. Some studies have evaluated for lumbar stenosis as a whole without the distinction of foraminal stenosis. Others do not clearly delineate a positive Kemp or quadrant test finding in which a positive test may include either the presence of lower back pain or radicular symptoms in a lower extremity. Lyle et al. [21] studied the relationship of physical examination findings in individuals with degenerative lumbar conditions and reported that 70% (n=42) of patients with lumbar stenosis had a positive Kemp test. However, it is important to note that this study did not differentiate the type of lumbar stenosis that patients had and defined the positive quadrant test as either back pain or the presence of radicular symptoms. In evaluating individuals who underwent posterior lumbar interbody fusion, Watanabe et al. [22] reported that 79% of their subjects (n=15) had a positive Kemp sign but they did not further describe their criteria for a positive test. Dobbs et al. [23] evaluated a modified lumbar extension test with 2 components to evaluate the validity of a symptomatic response physical exam maneuver in diagnosing either central or foraminal lumbar stenosis seen on MRI in a small sample of 30 individuals. One component of the test consisted of lumbar extension and side flexion held for 60 seconds. They found the mean time for symptom onset with the exam maneuver was 28.03 seconds for mild foraminal stenosis, 15.89±10.50 seconds for moderate stenosis, and 18.13±25.51 seconds for severe stenosis. However, there was no statistically significant association between evidence of foraminal stenosis on MRI with the extension and lateral flexion component of the test (r=0.09, p=0.62). Possible consequences of the small sample size and lack of statistical power of the study of Dobbs et al. [23] include increased likelihood of type II error and production of nonstatistically significant results.
Our current study differs from the study of Dobbs et al. [23] in 2 notable areas. First, this current study sustained the provocative maneuver for at maximum time of 10 seconds while study of Dobbs et al. [23] sustained the maneuver for 60 seconds. Findings of symptom onset in the study of Dobbs et al. [23] suggest that the duration of the provocative maneuver may be too short to capture all positive cases in this current study which consequently may have decreased the sensitivity of the test. Secondly, the study of Dobbs et al. [23] utilized MRI findings as the standard for detection of foraminal stenosis, whereas this current study utilizes both MRI and a SNRB. A prior study by Aota et al. [24] had found that MRI imaging on its own has a false-positive rate of approximately 30%–40% when diagnosing lateral lesions of the lumbar nerve roots. Regarding the validity of diagnostic lumbar SNRB, Yeom et al. [20] found that diagnostic lumbar SNRBs had a 77% PPV and 71% NPV in achieving pain relief greater than 70% in individuals with radiculopathy. Given this, a positive finding on physical examination in the Dobbs et al. study may not necessarily correspond to the pathological finding on MRI imaging. This current study combines pathology found on MRI and relief from a SNRB to confirm that positive findings on the Singh-Hartl maneuver are indeed a true positive finding.
There are several imitations of this study which must be acknowledged. The small sample size recruited for this study also possibly has an increased type II error rate with decreased statistical power. Additionally, when considering data from the prior study of Dobbs et al. [23], the duration utilized for the Singh-Hartl maneuver in this study may not have been long enough to capture additional cases of foraminal stenosis; this is worth examining in the future. Furthermore, future research endeavors may seek to further characterize the degree of foraminal stenosis on MRI imaging in relation to onset of symptoms with the Singh-Hartl maneuver and the outcome of SNRB. Altogether, given the study’s findings, its limitations and avenues for future research validate the examination maneuver, clinicians should continue to use symptomatology, multiple physical examination maneuvers, and imaging modalities to guide diagnosis of lumbar foraminal stenosis.
CONCLUSION
Evaluation of the validity in using proposed Singh-Hartl maneuver, which represents a modified Kemp maneuver, to diagnosed lumbar foraminal stenosis shows some promise in providing a diagnosis of lumbar foraminal stenosis. The maneuver exhibits moderate strong ability to rule-in the diagnosis and a moderate ability to rule out the diagnosis when using 75% improvement of radicular symptoms to diagnose lumbar foraminal stenosis.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.