Spontaneous Pneumorrhachis With S1 Radiculopathy: Current Evidence Regarding a Rare Presentation
Article information
Abstract
Degenerative disc disease (DDD) causes the vacuum phenomenon, resulting in the accumulation of air in the disc space. Pneumorrachis refers to the presence of air in the spinal canal, which is sometimes associated with DDD. Air may form pseudocysts and compress the root or thecal sac, causing symptoms similar to those of disc herniation or an osteophyte. Pneumorrachis, which is a rare condition, needs proper preoperative magnetic resonance imaging and computed tomography for an accurate diagnosis and differentiation from other pathologies, such as a discal or facetal cyst. The pathogenesis of pneumorrhachis remains unclear, and treatment guidelines have not been established. Asymptomatic cases need observation. Symptomatic cases may require percutaneous aspiration, which can result in incomplete pain relief and high recurrence rates. Open or microsurgical decompression and excision provide relief, but very few reports have described the role of full-endoscopic management for this condition. Herein, we report the case of a 78-year-old woman with an epidural gas-containing pseudocyst and left-sided S1 radiculopathy, who was managed with endoscopic decompression and excision of the cyst. The case highlights the need for thorough imaging and the establishment of standard guidelines for diagnosing and managing such cases. Endoscopic techniques offer better visualisation and manipulation, leading to significant improvement in symptoms. Advances in endoscopic techniques have made it easier to perform lumbar surgery through the endoscope, making it a viable option.
INTRODUCTION
Accumulation of air in tissues can result from trauma, infection, surgery, or other causes, leading to conditions such as pneumothorax, pneumomediastinum, pneumocephalus, emphysema, and pneumatocysts [1]. These conditions can range from minor to potentially fatal symptoms, depending on their location and severity. The accumulation of air in the spinal canal is referred to as either extradural (pneumorrhachis, pneumocele, epidural emphysema, etc.) or intradural [2] (pneumomyelia). Any mass effect may cause neurological symptoms to manifest [3].
Air in intervertebral disc spaces is often associated with degenerative disc disease (DDD) and fractures, appearing like phantom nuclei and intradiscal vacuum phenomena. This instability-related phenomenon occurs when bone endplates move due to instability, resulting in the development of nitrogen, oxygen, and carbon dioxide and creating vacuum or air gaps [4]. Kummels disease manifests as black shadows in the vertebral body when fragments of broken vertebral bone distract, leaving empty spaces of air. While gas collection in the vertebral/disc space is not very significant clinically, air in the spinal canal/foramina might cause greater discomfort [5].
Cystic lesions in the spine occur due to mucin-filled facetal, ganglion and synovial cysts that are slowly growing and linked to end-late osteophytes and facetal arthritis [6]. Intraspinal gas-containing pseudocysts may arise spontaneously in DDD, trauma, infection, or intervention [7]. Symptoms include greater discomfort when standing and walking due to underlying degeneration in DDD. Symptoms of radiculopathy arise when the cyst in the spinal canal or neural foramina compresses a root, resembling a herniated disc or osteophyte [7].
Gas-containing cysts are easily misinterpreted as conventional herniated disc material, so careful examination of T1 and T2 magnetic resonance imaging (MRI) sequences is necessary to distinguish disc herniation from an air-filled pseudocyst radiologically. Some authors suggest surgical removal for symptomatic gas-filled pseudocysts. Standard operative management for gas-containing cysts includes open or tubular minimally invasive decompression by laminectomy for radiculopathy and canal stenosis, similar to disc herniation or canal stenosis.
The prevalence of pneumorachis or air-filled cysts in the spine is still unknown, but recent literature has shown an increase in asymptomatic, symptomatic cases with degenerative spinal diseases, and iatrogenic cases after percutaneous interventions or after spinal surgery. Few cases have been described in the literature presenting with radicular and neurological symptoms managed by percutaneous interventions. Endoscopic spinal surgery, being associated with less soft-tissue damage and perioperative morbidity, has become the gold standard for treating a variety of lumbar spine disorders.
We present here a rare case of symptomatic intraspinal gas-filled pseudocyst with DDD and vacuum disc phenomena at the L5–S1 level and S1 radiculopathy managed by endoscopic decompression of the root and excision of the cyst. We also made an attempt to review the literature reporting similar cases, emphasising the role of radiology in diagnosis and managing them with minimally invasive techniques.
CASE REPORT
1. Clinical Presentation
A 78-year-old female with mild axial low back pain reported increased radiating pain to her left calf for 1 month. She had no prior history of trauma, fever, or bronchial asthma. She was using apixaban for prophylactic anticoagulation for moderate coronary artery disease and had no recent symptoms. She had surgery for a stomach ulcer 40 years ago and cataract surgery 7 years ago, with no current problems. As pain increased, she needed support for ambulation, and radiating pain worsened with posture changes, standing, and walking.
Radiating pain followed a dermatomal pattern along the S1 dermatome. Local tenderness was present along the L5–S1 midline region, with movements causing a greater increase in radiating pain than axial back pain. The straight leg raising test was positive on the left side at 45° and negative on the right side. Her back pain visual analogue scale (VAS) score was 5/10, and the severity of radiation to the left lower limb was 9/10. No sensorimotor symptoms were found.
2. Investigations
Laboratory tests revealed a normal leukocyte count, erythrocyte sedimentation rate, renal, and liver functions. Cardiac screening revealed a 58% ejection fraction with mild left ventricular hypertrophy and grade 2 diastolic dysfunction. Spine imaging showed marginal vertebral osteophytes. Dynamic x-rays showed no instability. Computed tomography (CT) and MRI scans were performed. MRI was showing a degenerate L5–S1 disc with a cyst-like structure, equally hypointense in T1 and T2, encroaching onto the thecal sac and traversing the root towards the left side (Figures 1 and 2).
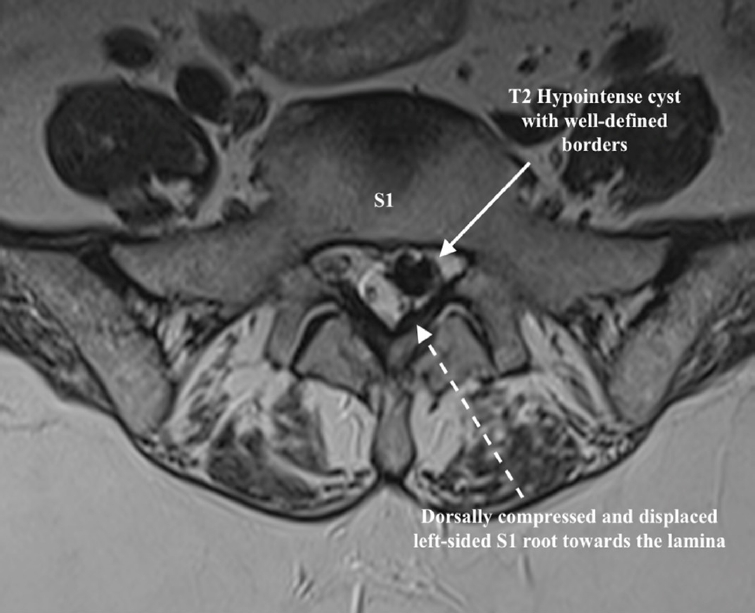
Axial T2-weighted magnetic resonance imaging showing a hypointense cyst compressing the left side of the traversing root.
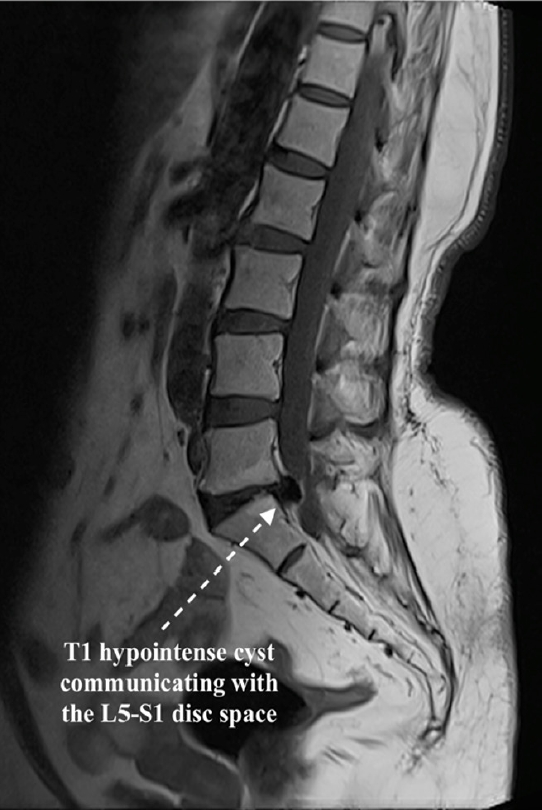
Sagittal T1-weighted magnetic resonance imaging showing a hypointense cyst communicating with the disc space with endplate Modic changes.
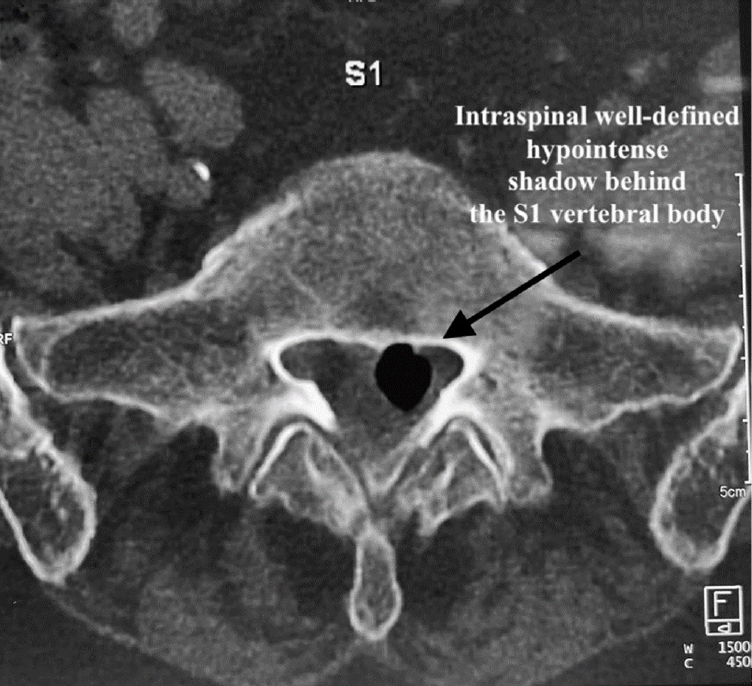
Endplate type II Modic changes were seen in T2 images at L5–S1 levels. A CT scan (Figures 3 and 4) confirmed a clear presence of air-filled space in the intervertebral space, with a hypodense or black cyst-like structure lying in the intraspinal canal more towards the left side with probable compression on the left-sided neural structures.
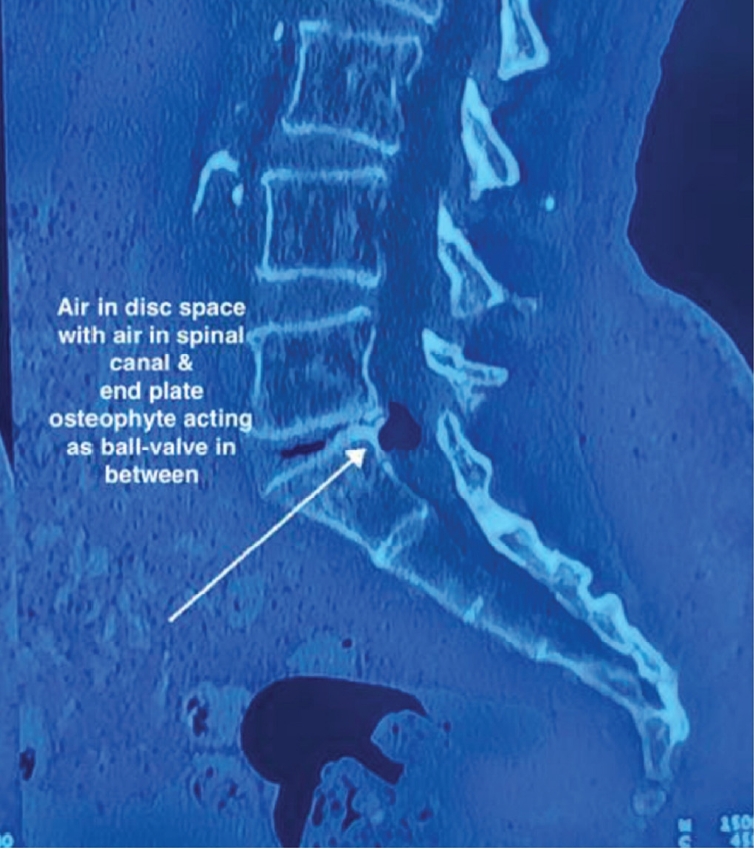
Sagittal computed tomography with a vacuum disc and cyst, as well as a disc osteophyte, creating a ball-valve effect.
Operative decompression was planned for S1 radiculopathy by the interlaminar endoscopic method with a single portal technique.
3. Procedure, Findings, and Outcome
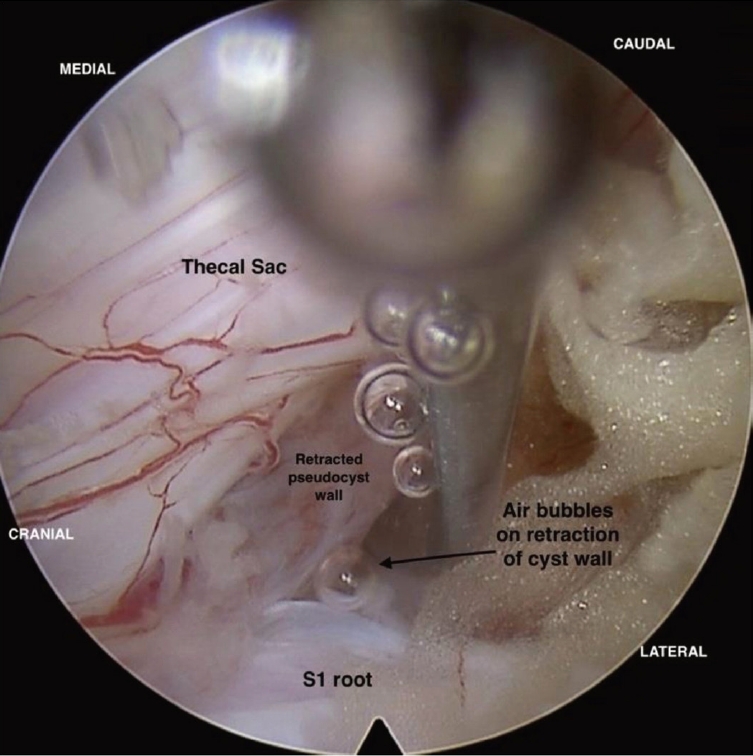
Under general anaesthesia, a small 8-mm portal was made at the L5–S1 interlaminar window, 1 cm lateral to the spinous process, and a 30° interlaminar endoscope was placed over the space. Partial laminectomy was done, and ligamentum flavum was dissected from the laminar margins with a dissector slowly and partly excised. A cyst-like structure with a well-defined wall was seen between the thecal sac and the left-sided traversing root S1 (Figure 5). The cyst located in the axillary recess of the S1 root was splaying the S1 traversing root laterally, causing significant compression on it.
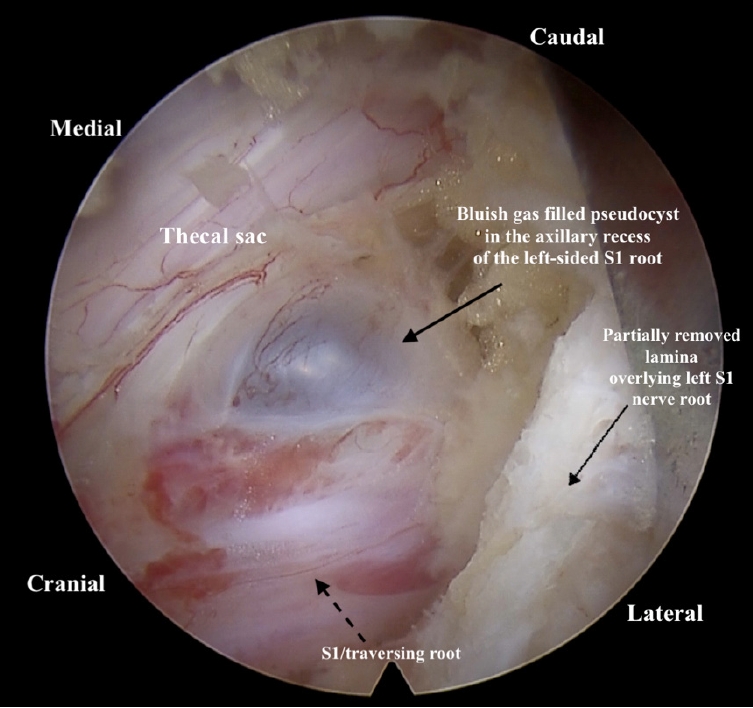
Intraoperative endoscopic image after partial resection of the lamina, with the cyst lying in the axillary recess of the left S1 root.
Careful dissection of the cyst wall was done from the thecal sac and traversing root. The cyst was soft in consistency and compressible with the dissector, appearing sightly bluish compared to surrounding tissues. When the cyst was mobilized, air bubbles escaped continuously through the crevasse, ending with the cyst's collapse (Figure 6). The cyst was slowly re-expanding immediately, probably due to the fluid medium used during endoscopy surgery.
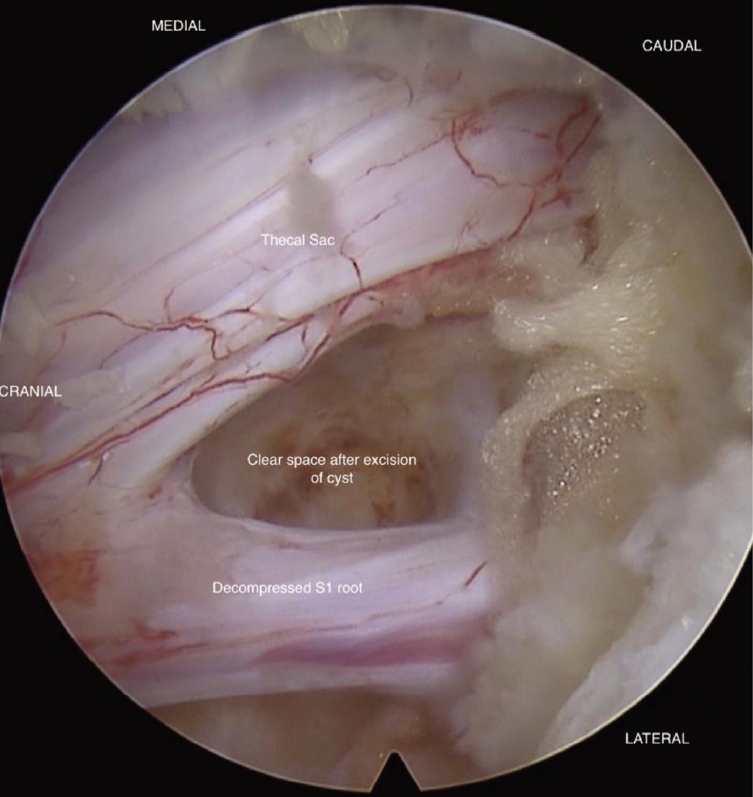
An attempt to puncture the cyst wall for a discogram was not possible as it was too thick to be pierced with a needle. The cyst wall was excised, and tissue was sent for histopathological examination. No obvious disc material was seen, and the cyst wall was likely stretched posterior longitudinal ligament (PLL)/annulus tissue, forming pseudo-capsule around the trapped air. Adequate decompression of the S1 root was appreciated after the excision of the cyst, with no other structure compressing the neural structure (Figure 7).
As the patient had predominant radicular pain with limited axial pain, only decompression was done, with no attempt for fusion or fixation of the affected level.
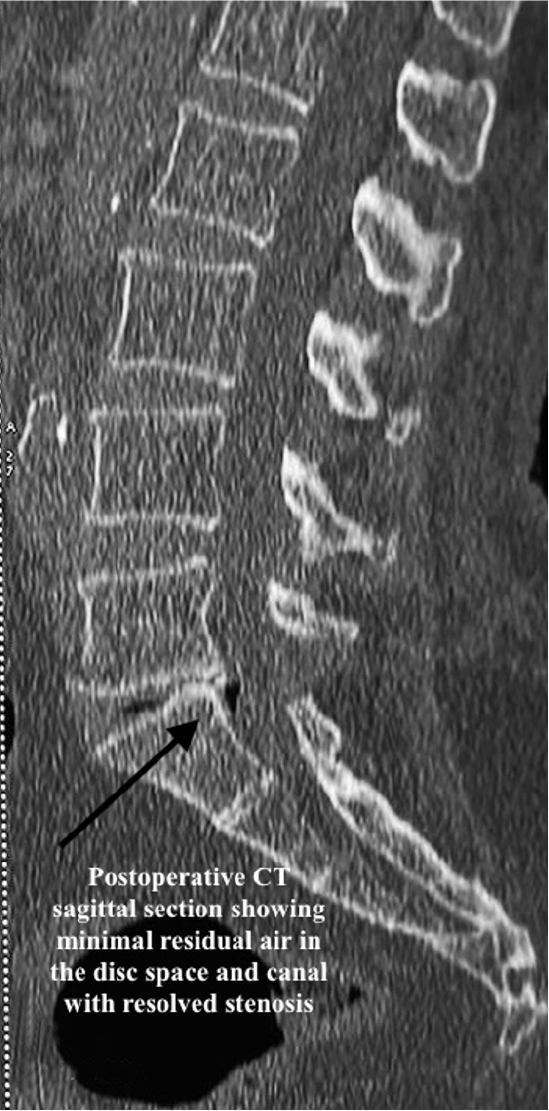
Postoperatively, the patient had significant improvement in pain, with VAS axial pain improving to 2/10 and VAS leg pain improving to 0/10. She had complete relief from radiation pain. Postoperative CT (Figure 8) and MRI (Figure 9) demonstrated complete removal of the intraspinal air-filled pseudocyst and decompressed neural elements. Mild residual air in disc space was suggestive of the vacuum disc phenomenon (Figure 10) and ongoing degenerative spine disease. The histopathology of the tissue from the cyst wall was showed fibrous connective tissue. At the 6-month follow-up, the patient had no recurrence of symptoms and minimal axial back pain, which was not disturbing her daily functional activities.
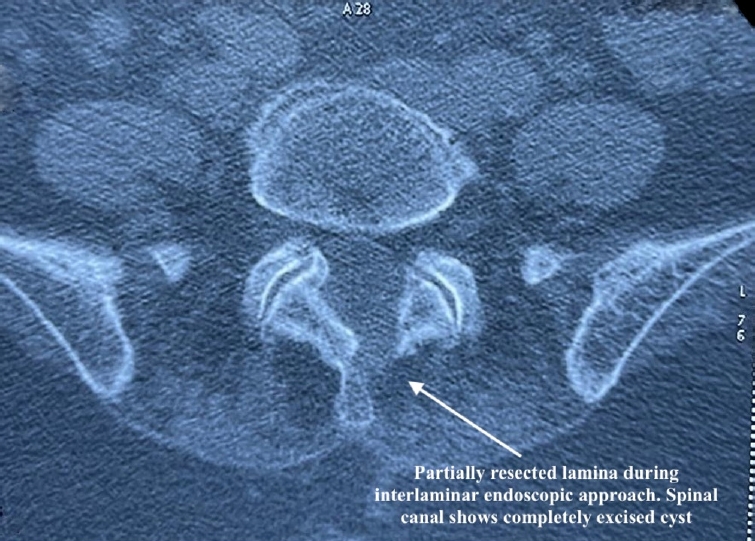
Postoperative computed tomography showing resolution of the air shadow and partial removal of the lamina.
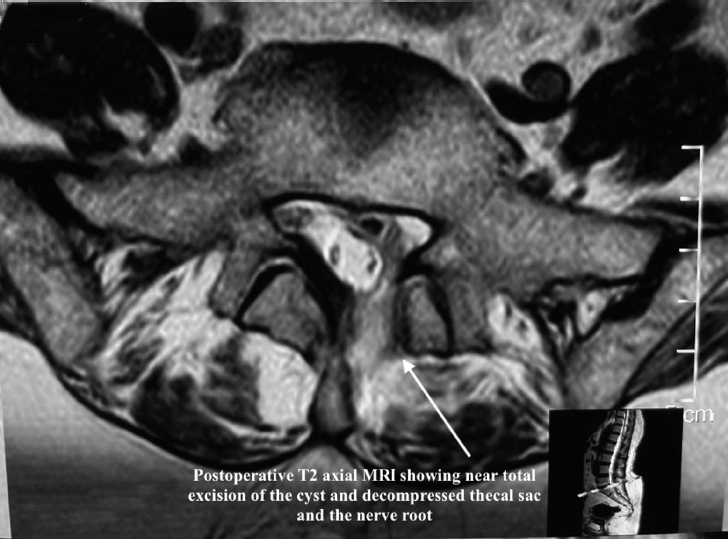
Postoperative magnetic resonance imaging (MRI) showing decompression of the root and negligible residual air and oedema along the endoscopic portal tract.
4. Ethics Statement
Written and informed consent was taken from the patient for publication. Patient was assured of anonymisation of intraoperative images and radiological images respecting the right to privacy.
DISCUSSION
1. Etiopathology and Symptomatology
The finding of gas within the disc space, referred to as the vacuum disc phenomenon, was first described in 1937 by Magnusson. Ford et al. [8] performed chromatography analysis of the gas aspirated after discography and chemonucleolysis and found it to contain up to 92%–95% of nitrogen. Yoshida et al. [9] reported 3 cases with gas chromatography analysis of the air aspirated from the cyst and showed predominant nitrogen levels higher than atmospheric levels.
Air in cervical spine x-ray was first described by Gordon et al. [10] in 1977, called pneumo-myelogram in cervical spine along with pneumocephalus due to head injury. Multiple reports were presented with traumatic aetiology and named as pneumocele, epidural emphysema, spinal/epidural pneumatosis, pneumosaccus and air/pneumo-myelogram [1]. As the literature enlightened more similar cases over the years and the availability of CT, eventually cases of nontraumatic presence of air in the spinal canal were reported and gave different nomenclature. The presence of gas-filled lesions in the lateral recess of the lumbar spine was first described in 1984 by Gebarski et al. [3] among symptomatic people identified in CT scans, and they were relieved by aspirating the gas. The term pneumorachis was coined by Newbold et al. [11] in 1987.
In 1994, Cheng et al. [12] reported similar cases of radiculopathy where they identified air inside the spinal canal through CT scans. Subsequently, asymptomatic cases have been reported, like pneumorachis in chronic obstructive pulmonary disease (COPD) cases and polytrauma cases identified incidentally [11]. Hamman syndrome, a spontaneous pneumomediastinum with intraspinal air in the entire spinal cord, was also reported but asymptomatic. Chronic cough in asthma and COPD cases resulted in air spaces in the intraspinal canal without spinal symptoms. Cysts may remain asymptomatic or present with radicular or neurological complaints, depending on the location of the cyst and the compression of neural structures.
Cyst-like lesions in the spine, described by Chiba et al. [13], were differentiated from similar-appearing lesions based on the content of the cyst as seen on imaging, like synovial/facet, ganglion, discal and perineural cyst. They described them as a separate entity of problems, presenting similar to disc herniation, but as fluid-containing lesions. Facetal/synovial cysts communicate with and adhere to facet joints and are filled with mucinous material. Air-filled cysts differ from these lesions, generated from and supposedly communicating with the intervertebral disc space.
Epidural gas-containing cysts are often asymptomatic and coexist with sequestrated disc fragments. Isolated air-filled pseudocysts without disc herniation causing compressive symptoms are rarely reported. The underreporting of these lesions could be due to diagnostic dilemmas in differentiating them from simple disc material in MRI or surgeons relying less on CT for imaging. Air-filled cysts can be accurately identified using T1 hypointense signal material and CT as an additional imaging modality. Additionally, during open/MIS surgery, it is difficult to distinguish these cysts from simple disc bulges, as endoscopic surgery captures air distinctly.
The pathogenetic mechanisms behind intraspinal gas-containing pseudocysts, which cause lumbar radicular discomfort, are not well understood. Instability or degenerated discs can cause end plate permeability changes, leading to gas collection in the intradiscal space. Gohil et al. [4] proposed a theory explaining this, stating that an expanded disc or joint under axial pressure creates negative intradiscal or joint pressure (Boyle’s law) and decreases gas solubility in surrounding tissues (Henry’s law), leading to gas bubble formation until equilibrium pressure is reached, resulting in the vacuum phenomenon, which is applicable to degenerative osteoarthritis of major joints [14].
Air herniation in the spinal space can occur due to axial pressure on the disc space and rupture of the annulus or PLL, causing pressure on the root or thecal sac. Air may be trapped below the PLL due to the ball-valve effect, causing a mass effect. However, air, being soft, cystic, and compressible, usually causes fewer symptoms.
Liu et al. [15] classified gas inside the lumbar spinal canal into 4 types. They named them air cysts, pseudocysts lined by tissue, air-containing disc herniations and honeycomb patterns. Accordingly, the present case was classified as a pseudocyst pattern. Similar to some disc herniations showing pain out of proportion to their size, air-filled cysts might cause symptoms depending on the location [16]. The symptomatic presentation was probably considered to be due to the air-filled cyst expanding from disc space and remaining expanded due to the ball-valve mechanism at the endplate annulus junction forming as a space-occupying lesion lying in the canal, causing pressure symptoms.
2. Evaluation and Differential Diagnosis
Multiple authors have reported symptomatic air-filled cysts as a separate entity, that requires equal attention for prevention and management [15]. CT is the gold standard for diagnosing vacuum phenomena and identifying the extent of air as well [4]. Thorough MRI differentiation is needed to identify hypointense lesion in T1 and T2 imaging. A CT and MRI combination is ideal to identify the actual prevalence of this entity [4].
Apart from trauma and degenerative spondylitis, another cause of air in the spinal canal being reported in recent years is iatrogenic or postintervention. Few cases of percutaneous procedures like lumbar puncture and epidural injection were reported. With increased management of radiculopathy due to disc herniation or stenosis by root blocks or epidural injections, post injection formation of air-filled cysts also cause cord and root compression [17] and neurological sequelae have been reported [18]. Postoperative cases of air in spinal canal have recently been presented as case series, showing a new possible aetiology of persistent pain during postoperative differentiating from haematoma as a cause of residual compression of neural structures. Spinal surgery can be a cause of intraspinal epidural gas formation, with recently reported postoperative symptomatic cases after microdiscectomy [19,20].
3. Treatment Plan and Surgical Management
Asymptomatic lesions need observation. Symptomatic lesions need complete excision for complete relief as well as to prevent recurrence. Transforaminal or selective nerve blocks aid in confirming the pain generator to plan surgical excision. CT-guided aspiration may be reserved for palliative or pain relief options with a risk of recurrence. Percutaneous management of an air-filled cyst with radiculopathy were reported as summarised in Table 1. CT or fluoroscopic-guided aspiration of the air was done, with a reported relapse of symptoms and requiring surgical decompression [21,22]. Some cases managed by nerve block/CT-guided aspiration showed resolution in symptoms and follow-up imaging [23-25]. Impiombato et al. [26] reported aspiration with an angiographic catheter passed through sacral hiatus for L5 radiculopathy. Slow and delayed recovery with nerve block alone, but long term relief with resolution in CT was reported by Kim et al. [27]. As percutaneous decompression of the cyst, has shown recurrences, excision of the cyst for adequate decompression was found to be essential.
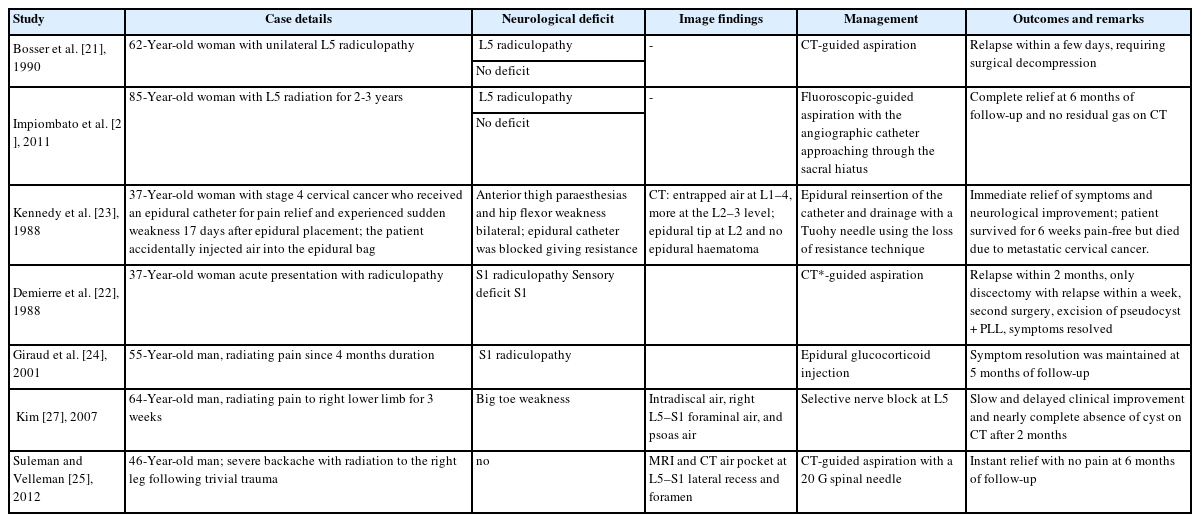
Reported cases of symptomatic epidural pneumorrachis managed by percutaneous imaging-guided aspiration
The choice between percutaneous/open and endoscopic decompression is debated, as some cases reported incomplete removal due to limited access. Theoretically, decompression alone does not prevent recurrence, as ongoing degenerative processes may perpetuate air or intradiscal changes, but the literature is scant to support this. Open decompression and laminectomy were reported for symptomatic cases, and gross neurological deficits were evident for adequate decompression. Open surgical treatment included standard/microsurgical total/hemilaminectomy and cyst excision for decompression and fusion in selected cases, which were eventually shown to have relief with no recurrence [2,24,28-35]. Most lumbar cases were managed with decompression alone. A case operated on for cervical intraforaminal air cyst and radiculopathy was managed by decompression and fusion [35]. Air causing cervical myelopathy was managed by decompression alone [34].
Endoscopic spinal surgery has become one of the standard treatments for degenerative lumbar diseases. It has shown many surgical advantages, such as small skin incisions, less soft tissue and bone trauma, and fast postoperative recovery. A varied spectrum of cases, from simple disc prolapse to highly migrated disc herniation, foraminal stenosis, and canal stenosis, are now being treated with full-endoscopic surgery using various accesses and techniques. Endoscopic decompression was done for similar cases, with only 4 reported similar cases (Table 2) in the last few years. Zhu et al. [36] and Inokuchi et al. [37] used transforaminal access for L5–S1 level. Chen et al. [38] used interlaminar access at L5–S1 level similar to present case. An and Lee [39] used UBE access at L4–5 level.
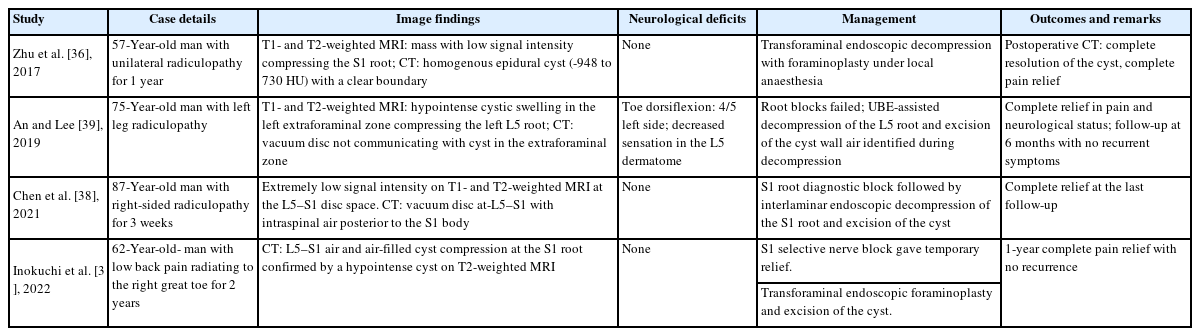
showing previously reported epidural air-filled pseudocysts managed by endoscopic decompression and excision of cyst
The gas-containing cyst wall is usually thin and bursts easily, making it difficult for operators to identify the exact pathology. In this case, the cyst could be exposed without premature rupture due to careful dissection with a magnified endoscopic view. The diagnosis of the gas-containing cyst was confirmed by bubbles from the cyst wall when incised and pushed with a probe. Endoscopic decompression surgery offers benefits such as easy discrimination of anatomic structures, delicate manipulation of pathology, and detailed operative information, contributing to the successful outcome in this case.
When air bubbles escape, the cyst collapses but may again expand back to its normal size due to residual air or fluid entering the cyst space. There are reports of the recurrence of cysts after image-guided aspiration, suggesting that the gas generated from intradiscal space will continue to accumulate. This also supported our intraoperative observation of cyst getting expanded after air evacuation. There is a need to study dynamic imaging in these sorts of cases to see and confirm the impact of axial load on the gas movement in disc space and in relation to the spinal canal. Further investigations are needed to confirm these speculations.
Postoperative air-filled cysts have been increasingly reported, causing recurrent symptoms after surgery and possibly explaining residual pain in failed back cases [20]. Imaging is only used in refractory cases with persistent and residual pain. With limited evidence from reported cases, understanding the pathogenesis and clinical manifestation of these cysts is crucial for planning treatment approaches. A comprehensive classification is needed to guide treatment approaches.
4. Recurrences
Complete removal of the cyst wall is essential to prevent recurrence. Some authors recommended excision of PLL to prevent this complication [20,40]. The recurrence of air-filled cysts managed by decompression, causing similar symptoms, has not been reported in the literature yet. With the latest advent of endoscopy techniques, minimally invasive decompression and excision of the cyst are feasible options with better outcomes. Fusion of the level for ongoing degenerative disease or instability if present is indicated as per the surgeon's choice and patient characteristics. More cases and follow-ups are required to formulate guidelines for similar cases.
CONCLUSION
The vacuum phenomenon or DDD must be carefully assessed for the presence of air-filled cysts causing symptoms. The size and location of the cyst are relevant for causing neurological symptoms and guide the treatment aspect in approach to decompression. Adequate decompression of cyst can be done by image-guided intervention with chance of relapse and complete relief is possible with excision of the cyst by percutaneous or open procedure. Endoscopy offers a minimal invasive option in decompression of such presentations without a need for fusion/radical procedure and cyst excision can be accomplished completely.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.