Biportal Endoscopic Transforaminal Lumbar Interbody Fusion With Percutaneous Instrumentation: A Technical Note
Article information
Abstract
Endoscopic spine surgery (ESS) has gained popularity in recent years due to its effectiveness and minimal invasiveness. In addition to decompression surgery, ESS has been used in lumbar fusion surgery to minimize tissue trauma and improve the fusion rate due to its ability to facilitate better endplate preparation under an endoscope. Biportal endoscopic transforaminal lumbar interbody fusion (BETLIF) has recently been proven to be a safe and efficient method with good clinical outcomes. We have adopted BETLIF to treat patients since 2021 and have experienced satisfactory results. In this article, we introduce our surgical steps and techniques for BETLIF with percutaneous instrumentation.
INTRODUCTION
Lumbar interbody fusion is considered as the treatment of choice for treating patients with unstable degenerative lumbar disease in decades [1-3]. Among the various minimally invasive (MIS) methods, MIS transforaminal lumbar interbody fusion (MIS-TLIF) has become a popular method. MIS-TLIF possess advantages of direct neural elements decompression, simultaneous instrumented fusion with minimized trauma to surrounding structures [4-7]. As the evolving of endoscopic spine surgeries recently, endoscopic lumbar interbody fusion (Endo-LIF) has proved to be a safe and efficient method with comparable clinical outcome to MIS-TLIF [8-14].
In our institute, we have adopted biportal endoscopic transforaminal lumbar interbody fusion (BETLIF) with percutaneous instrumentation since 2021. In this study, we introduce our surgical technique of BETLIF and discuss the advantage and pitfalls of BETLIF.
SURGICAL TECHNIQUE
1. Anesthesia and Positioning
All the patients received intubated general anesthesia with prone position over Relton-Hall frame with careful padding over chest, pelvis and knee. The C-arm fluoroscopy and endoscopic monitor was placed at the contralateral side to the operator.
2. Determination of Direction of Approach (Right or Left?)
The direction of our approach depends on which side of patient’s symptoms (leg pain) more predominant. For example, if patient sustained predominant right leg pain, we choose right-side approach.
3. Skin Incision
We use the incisional wound for percutaneous screws as the wound for biportal scope surgery. That is, we combine the wounds for screws and scope. Two ipsilateral skin incision is made over center of trajectory line of upper and lower pedicle on the anteroposterior projection of C-arm fluoroscope (Figure 1A). In short, the location of skin incision was at where the screws are.
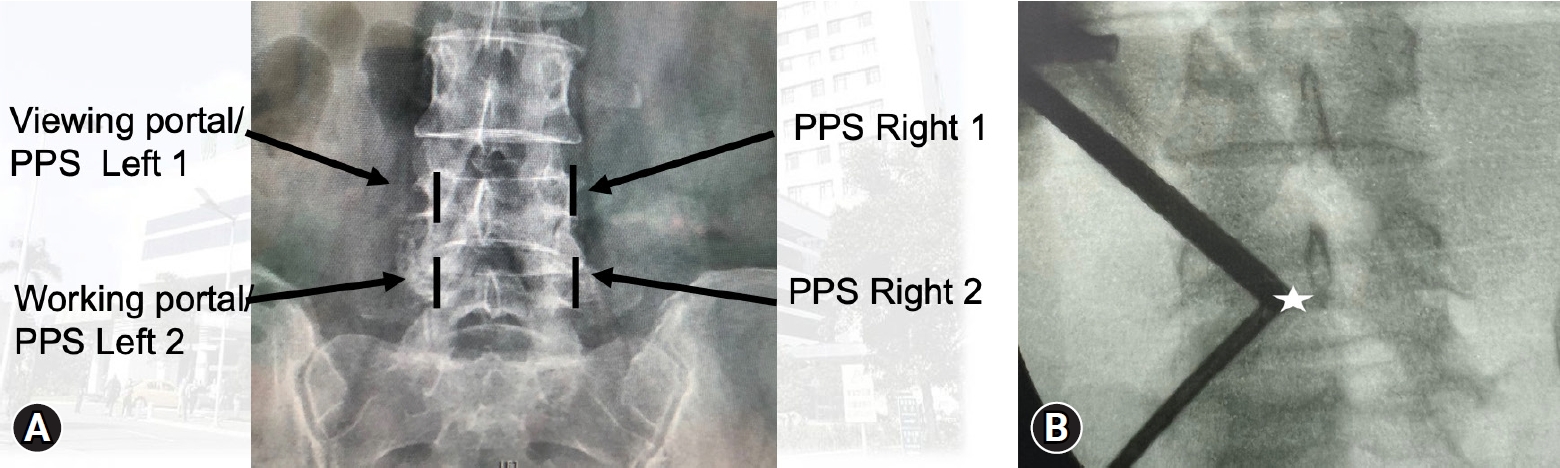
(A) Skin incision over a fluoroscopic anteroposterior view (left-side approach). Two skin incisions (black line) are made over the center of upper and lower pedicle. The upper one is used for viewing the portal and percutaneous pedicle screw (PPS) insertion, while the lower one is used for the working portal and another PPS. (B) Triangulation of biportal endoscopic surgery; the docking point is at the left spino-laminar junction (white star).
4. Docking and Establishment of Triangulation
For left -sided approaches, left hole was used as viewing portal while right hole as working portal. Following skin incision, a periosteal elevator was gently used to detach the muscle from lamina surface. Then the scope was inserted into left hole with the normal-saline irrigation system. A specialized sleeve was introduced into the working portal to maintain the outflow. A radiofrequency probe (RF probe) was introduced via working portal to clear the soft tissue as well as bleeding control. Thus, the triangulation of biportal endoscopic surgery with smooth outflow was established (Figure 1B).
5. Facetectomy and Auto-Bone Graft Harvesting
Our first docking point is left spino-lamina junction, second point is facet joint. Determining the docking points is helpful for those surgeons who are not familiar with unilateral biportal endoscopic (UBE) surgery and we could realize the surgical anatomy under endoscope more easily. Inferior articular process (IAP) facetectomy was done first with osteotome and high-speed burr (Figure 2A). The IAP was removed piece-by-piece under scope and harvested preparing for bone grafting. After IAP facetectomy, the superior articular process (SAP) was exposed. The upper half and lateral border of SAP was removed by burr and Kerrison punch.
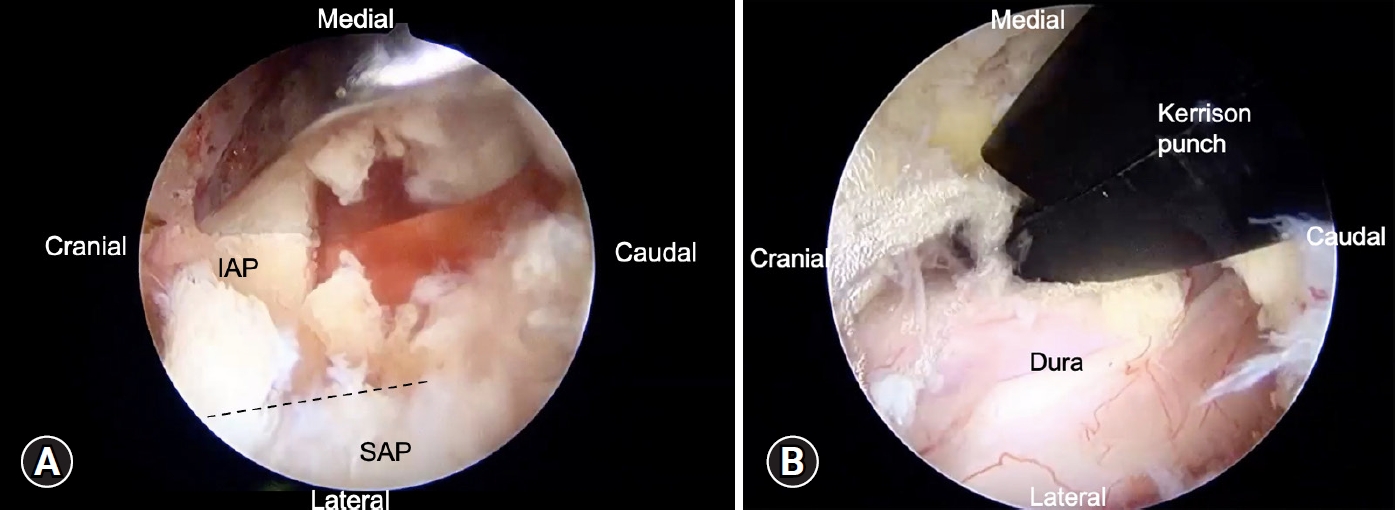
Endoscopic view of biportal endoscopic decompression surgery. (A) Facetectomy of the inferior articular process (IAP) piece-by-piece of the left lamina using an osteotome. (B) Bilateral decompression with the over-the-top approach, showing a well-decompressed dura sac. SAP, superior articular process.
6. Unilateral Laminotomy and Bilateral Decompression
The ipsilateral lamina was removed by conventional Kerrison punch or high-speed burr, followed by removing ligamentum flavum. The contralateral decompression could be achieved by over-the-top approach [15] (Figure 2B). We do bilateral decompression for every patient.
7. Discectomy and Endplate Preparation
The disc opening at the Kambin triangle was explored and dura was protected by nerve retractor by an assistant (Figure 3A). Similar with MIS-TLIF, discectomy could be done by conventional pituitary rongeur and endplate curettes. Serial disc reamers will be used to release the anterior longitudinal ligament and the appropriate cage size was measured under C-arm fluoroscope.
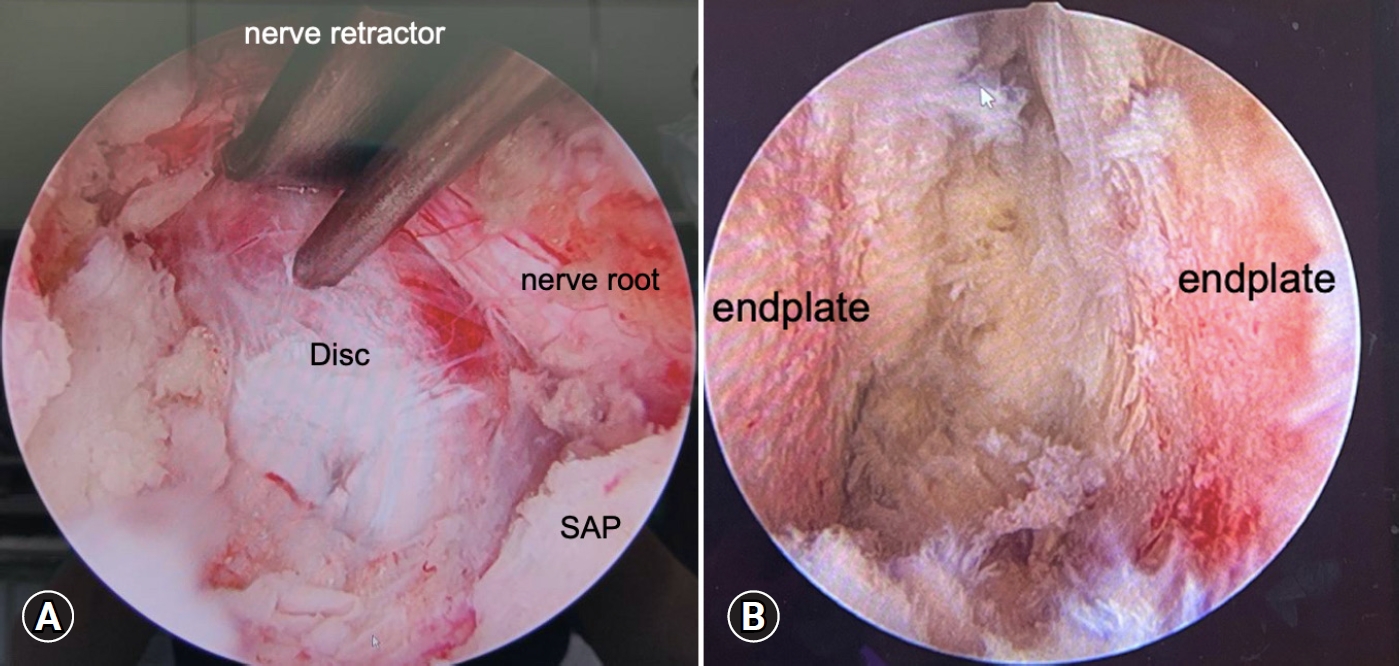
Endoscopic view of discectomy and endplate preparation. (A) The ipsilateral nerve root was retracted using a nerve retractor, and the disc opening was exposed. (B) The upper and lower endplates with subchondral bone were exposed after removal of fibrocartilage and disc materials. SAP, superior articular process.
To facilitate endplate preparation, the scope was advanced near the annulotomy site till we could observe the insight of disc space (Figure 3B). The remaining disc materials and cartilage-fibrosis tissue could be removed by instruments or RF probe.
After thorough endplate preparation, bone grafting and cage insertion is the final crucial step for interbody fusion. A bone-grafting funnel was inserted into anterior part of disc space after the water pump was closed (Figure 4A). The morselized bone graft harvested from IAP, SAP and lamina was impacted into the anterior disc space through the funnel. Besides, a putty-like artificial bone graft was packed into disc space. A bullet- shaped cage with bone graft at the central hole was inserted after the dura was retract by the nerve retractor (Figure 4B). The whole procedure of cage insertion could be monitored under the direct inspection. This is the most advantageous part of BETLIF compared with uniportal TLIF.
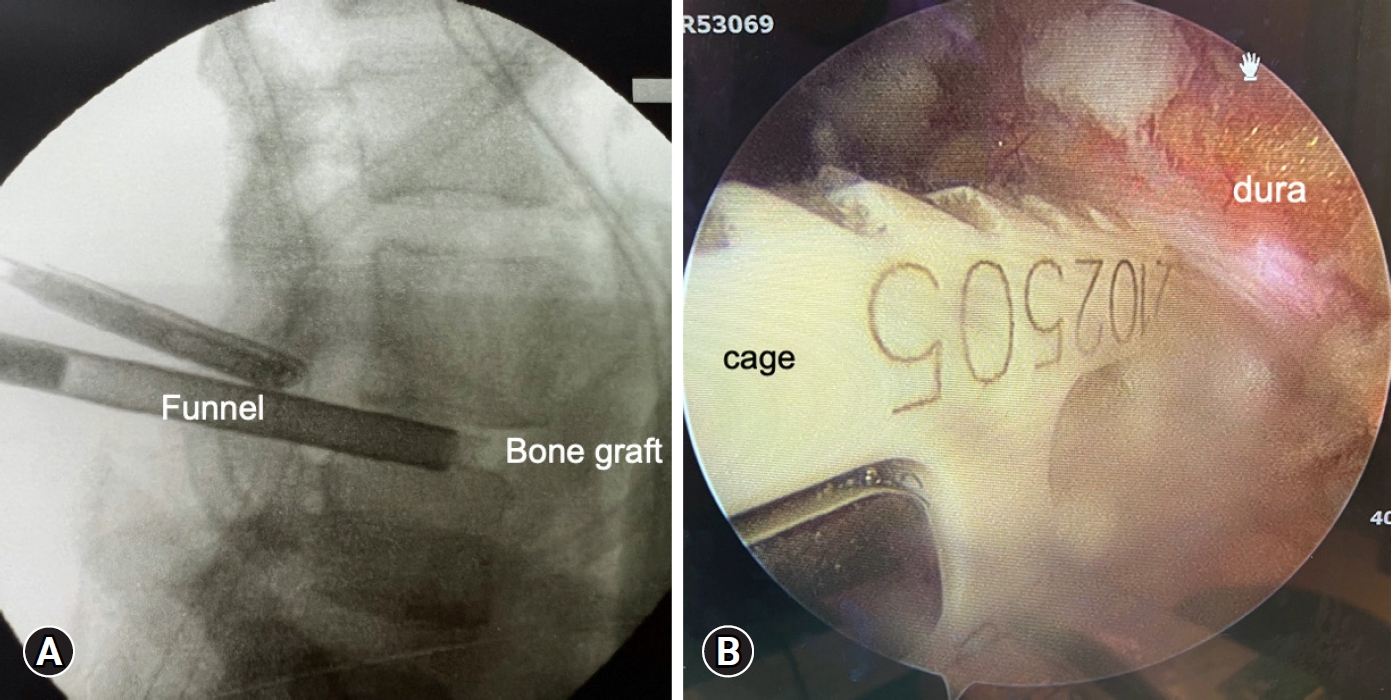
Bone grafting and interbody cage insertion. (A) Artificial bone mixed with morselized autologous bone chips were impacted into the anterior disc space through a funnel. (B) The interbody cage was inserted into disc space under full-time direct inspection via an endoscopic view.
The cage position was checked under the C-arm fluoroscope and was situated ideally at the anterior part of disc space.
8. Percutaneous Pedicle Screws Insertion Under C-Arm Fluoroscope
Two previously incised skin portals were used for percutaneous pedicle screws (PPSs) insertion. The other 2 contralateral skin incisions were made for contralateral screws insertion. After instrumentation completed, a 1/8 inch hemovac drain was placed, followed by skin closure by layers.
9. Postoperative Care
The patient was encouraged starting ambulation next day of operation. A corset was applied when sitting and walking.
INSTRUMENTS AND ENDOSCOPE
In our institute, endoscope we used in BETLIF is the same with ordinary arthroscope commonly used in knee arthroscope surgery (Figure 5A). We preferred 30° endoscope for its wider view under endoscope. Generally, the instruments used in BETLIF were similar with those used in MIS-TLIF surgery including Kerrison punches, pituitary rongeurs. The following instruments were useful in BETLIF including endplate curette (Figure 5B), nerve retractors (Figure 5C) and bone-grafting funnel (Figure 5D).
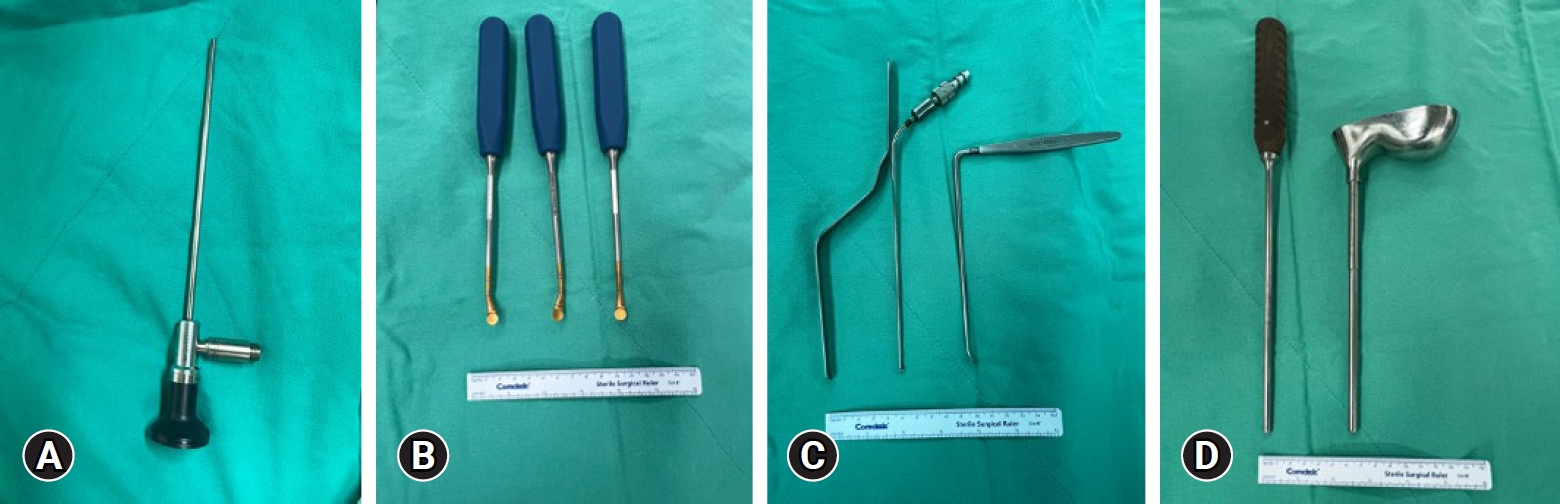
Introduction of endoscope and instruments for biportal endoscopic transforaminal lumbar interbody fusion at our institute. (A) A 30° knee arthroscope. (B) Endplate curette with 3 different angles for endplate preparation. (C) Nerve root retractors used for cage insertion to protect the nerve root. (D) Bone-grafting funnel with an impactor.
OUR CASE SERIES
Since June 2021 to July 2022, 12 patients including 8 females and 4 males underwent BETLIF in our institute by the authors. Among them, 11 patients sustained unstable lumbar spondylolisthesis and 1 patient was recurrent disc herniation. Operating level included 1 at L3–4, 6 at L4–5 and 5 patients at L5–S1. Average operation time was 105 minutes for scope time including decompression and cage insertion and 40 minutes for instrumentation. Complications included 2 patients with transient neuritis and one patient with wound eschar formation. At the minimal 1-year follow-up period, their back and leg pain improved.
CASE PRESENTATION
A 48-year-old female, who sustained chronic low back pain for 2 years. She felt pain when she bended or extended her back (mechanical back pain). Besides, she also complained of bilateral lower leg radiculopathy (originating from posterior thigh down to calf) as well as intermittent claudication (15 minutes). The radiographic of her lumbar-sacral spine revealed L4–5 slip with disc space narrowing. Dynamic flexion-extension x-ray also showed instability of L4–5 motion segment (rotation angle more than 10°) (Figure 6A). Magnetic resonance imaging (MRI) scan of her lumbar spine revealed L4–5 disc degeneration and bilateral lateral recess and foramen stenosis with spinal canal compromised (Shizas grade “B”) (Figure 6B).
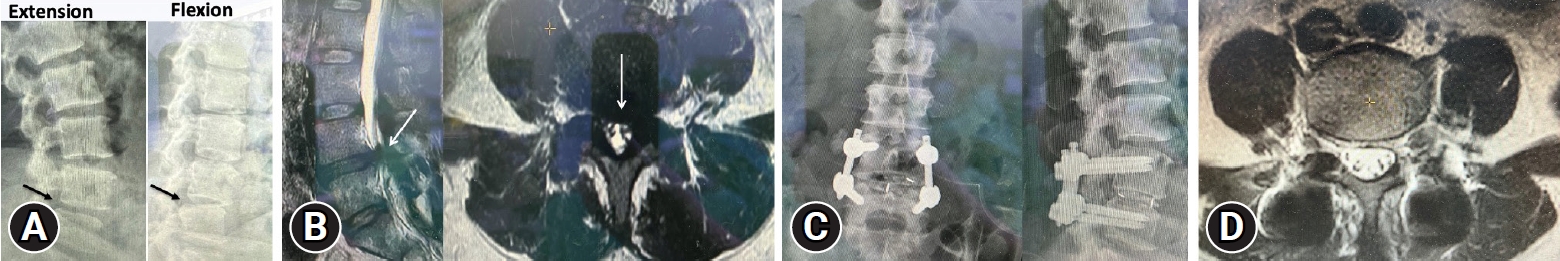
A 48-year-old woman experienced chronic low back pain and intermittent claudication. (A) Dynamic lumbar-sacral radiograph revealed unstable L4–5 motion segment (arrow). (B) Sagittal and axial view of lumbar spine magnetic resonance imaging (MRI) revealed remarkable stenosis at L4–5 (arrow). (C) Postoperative radiograph after L4–5 biportal endoscopic transforaminal lumbar interbody fusion revealed good implant position and well-reduced L4–5 vertebral bodies. (D) Postoperative axial MRI revealed good dura expansion.
She subsequently underwent L4–5 BETLIF and unilateral laminectomy for bilateral decompression for treating her unstable degenerative spondylolisthesis (Figure 6C). The surgery and postoperative course went smooth and she was discharged on third postoperative day. Her back pain and leg pain improved gradually as well as her walking endurance. At the 8-month follow-up period, she could ambulate well without low back pain except having minimal leg soreness and she was satisfied with the operation. Follow-up MRI revealed well-decompressed dura sac (Figure 6D).
DISCUSSION
In our opinion, BETLIF possess many advantages as a MIS fusion surgery of lumbar spine. They are familiarity, equipment – friendly, efficient and safety.
Basically, the procedure of BETLIF was similar with those of MIS-TLIF. While many surgeons used microscope performing MIS-TLIF, “endoscope-assisted” technique might be the major difference. As long as surgeons could be familiar with biportal endoscopic surgery technique, they could complete the BETLIF with the same concept of MIS-TLIF.
The basic concept of biportal surgery was that one skin incision served as “viewing portal,” the other incision was “working portal.” Surgeons could use conventional spine instruments through working portal instead of using special-designed tools. On the other hands, many special-designed tools were necessary to pass through the working channel of the endoscope when performing uniportal spine surgery. BETLIF was more equipment-friendly than uniportal TLIF.
Being beneficial from magnification and illumination of the modern endoscope system, surgical field under the biportal endoscopic surgery was light and clear. We surgeons could inspect surgical anatomy very well and remove lesions such as hypertrophic ligamentum flavum and osteophyte from neural elements safely. Because the endoscopic tip could proceed into epidural space, we could perform contralateral decompression without tilting table. Besides, we could do thorough “endplate preparation” under the clear scope view. Surgeons could look into disc space through endoscope to see if fibrocartilage was decorticated completely or not, which was difficult to achieve during open or MIS-TLIF. We think good endplate preparation is a key factor to achieve good interbody fusion.
Furthermore, we could obtain full-time and direct inspection under endoscope when inserting the interbody cage. This could minimize the risk of neural elements injury. We think this method is safer for patient and more comfortable for surgeons.
However, UBE-TLIF is technique demanding and there is certainly a learning curve. Before jumping into BETLIF, we suggest surgeons practicing biportal endoscopic discectomy or decompression firstly to familiar with basic techniques and smooth handling of scope and instruments.
CONCLUSION
In recent years, the indications of endoscopic spine surgery have been expanded from decompression-only surgery to fusion surgery. The technique of endo-LIF either using uniportal or biportal is still evolving. We think BETLIF with percutaneous instrumentation might be the least invasive fusion technique with many advantages currently. More BETLIF cases are needed to evaluate the clinical benefits and results.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
