AbstractObjective Chiari malformation type 1 (CM1) is a congenital hindbrain abnormality characterized by downward displacement of the cerebellar tonsils through the foramen magnum. The widespread accessibility of advanced technologies and imaging modalities has led to an increase in the popularity of minimally invasive (MIS) techniques in cranial and spinal pathologies.
Methods The study was conducted at a university hospital in Bogotá, Colombia. All data were obtained from the database of the hospital’s Neurosurgery Department. After institutional review board approval, the medical records of patients who underwent MIS posterior fossa decompression for CM1 were retrospectively reviewed.
Results Thirty-six patients underwent posterior fossa decompression through a minimally invasive approach during the study period. Nineteen patients met the inclusion criteria and were included in the data analysis. The patients’ chief complaints were headache (78.9%) and neck pain (57.9%). The average surgical time was 158.2 ± 50.5 minutes, with no significant difference in timing among different specialists. The most common postoperative complications were associated with dura closure, including 6 patients with pseudomeningocele and one patient with cerebrospinal fluid leak.
INTRODUCTIONChiari malformation type 1 (CM1) is a congenital hindbrain abnormality characterized by downward displacement of the cerebellar tonsils through the foramen magnum [1]. It is defined as herniation of the cerebellar tonsil below the foramen magnum of >3 mm in children and 5 mm in adults [2]. The most common symptom in both pediatric and adult population is pain or headache within the occipital and cervical regions [2].
These abnormalities have been associated with alterations of normal cerebrospinal fluid dynamics, which result in cerebellar and bulbar dysfunction symptoms [1]. Other related conditions include syringomyelia and hydrocephalus [2]. Syringomyelia is defined at magnetic resonance imaging (MRI) as the presence of single or multiple fluid-filled cavities within the parenchyma of the spinal cord [3]. After the advent of MRI, estimated prevalence of syringomyelia ranged from 1.9 to 8.4/100,000 [3-5]. About 50% of these patients have severe neurological damage and chronic progressive disability with complete loss of independence [3]. Prognostically speaking, even more unfavorable is the presence of syringobulbia in which swallowing and breathing bulbar centers are involved [3]. Surgery is advised for the patients’ symptomatic control or in cases when the latter conditions are clinically evident [1].
The craniocervical junction represents a complex transitional zone between the cranium and the spine [6]. It is a biomechanical and functional unit comprising bone, ligament, and soft tissue housing the spinal cord, and critical neurovascular structures [6,7]. Proper knowledge and study of these anatomical structures allows the implementation of new techniques and approaches [7].
The widespread accessibility of technological and imaging advancements has led to an increase in popularity of minimally invasive (MIS) techniques in cranial and spinal pathologies. Compared to other open techniques, the possible benefits include smaller incisions and less tissue trauma with preservation of muscles and ligaments which are fundamental for spinal biomechanics and stability of the craniocervical junction. In the postoperative phase, MIS to the posterior cranial fossa is frequently associated with reduced bleeding, fewer infection rates, better pain management, and quicker recovery periods [1,8]. The safety and efficacy of the procedure could be jeopardized by the significantly smaller surgical corridors and limited operative field exposure. In the present study, we describe our technique, and report our 10-year experience of MIS posterior fossa decompression for CM1.
MATERIALS AND METHODS1. Study PopulationThe study was conducted at a single center in Bogotá, Colombia. All data were obtained from databases of the Neurosurgery Department of the hospital. After institutional review board approval, medical records of patients who underwent a MIS posterior fossa decompression for CM1 over a 10-year period (January 2012 to December 2022) were reviewed retrospectively. CM1 was defined as a downward displacement of tonsils 5 mm or more below the lower limit of the posterior cranial fossa. All MIS treatments were performed by two spine surgeons, who are experienced in performing a variety of MIS spinal procedures. Patients with other Chiari malformations, previous decompressive surgery, unstable craniovertebral junction requiring fusion, and cerebrospinal fluid (CSF) abnormalities requiring diversion procedures were excluded from this study.
2. Data CollectionBaseline patient information such as age, sex, and past medical history were collected. Relevant symptoms and symptom duration were recorded. Operative information regarding type of surgery, duration of surgery, and intraoperative bleeding were obtained from operative notes. Post-operative data such as length of stay, recurrence or onset of symptoms, clinical and radiological changes were obtained. Patients were stratified by Chicago Chiari Outcome Scale (CCOS) for assessing the surgical benefits ranging from 4 (severely incapacitated) to 16 (excellent outcome) [8].
All patients underwent a postoperative computed tomography (CT) scan the day after surgery. MRI was ordered for all patients at three months. Outcomes were evaluated at the last follow-up visit using the CCOS. Finally, the esthetic component of the incision was independently evaluated by 4 examiners using the Vancouver Scar Scale (VSS) ranging from 0 (barely notable scar) to 13 (Severely pathologic scaring) [9]. All significant complications were recorded and treated accordingly.
3. Statistical AnalysisStatistical analyses were performed using RStudio Desktop Software 2022 Version (Posit). Descriptive statistics were performed to determine means and standard deviations for the different variables.
4. IndicationIndications for surgery in patients with CM1 have been a motive of controversy, especially considering diagnosis depends on radiographic findings that may be incidental. In the setting of tonsillar herniation some clear indications for surgery include the presence of associated syrinx, spinal malformation, or development of neurological deficit secondary to brainstem compression [10]. A survey for the American Society of Pediatric Neurosurgeons demonstrated surgery is reserved for symptomatic patients while asymptomatic subjects are followed clinically and radiologically [11]. Patients with CM1 may display a variable constellation of symptoms including nausea, vertigo, and neck pain. Nonetheless, intractable occipital headache exacerbated by Valsalva maneuvers is the most common one [11]. The subjective nature of headaches and their multiple etiologies create considerable debate on operating patients with this sole condition. The impact on quality of life is crucial when deciding to operate on patients who only present with associated headache.
Minimally invasive surgery (MIS) is a surgical approach that utilizes small incisions, specialized instruments, and advanced imaging technology to access and treat the affected area. All patients treated in our study where symptomatic and had failed conservative treatment with medication and physical therapy. Patient selection is crucial for obtaining positive outcomes with minimally invasive approaches. The indications we use for deciding on a minimally invasive approach for CM1 include [1,8]:
1. Syringomyelia: CM1 is often associated with the development of fluid-filled cavities within the spinal cord called syrinxes. MIS approaches can be an effective treatment for syringomyelia, particularly in cases where the syrinx is small and located in the cervical or upper thoracic spine [3].
2. Younger patients: Minimally invasive surgery may be preferred in younger patients, as minimal bone removal and soft tissue trauma can help preserve the integrity of the skull and spine, which is important for long-term spinal stability [12].
3. Tonsillar herniation <20 mm: Patients with tonsillar herniation smaller than 2 cm may be good candidates for a minimally invasive approach, as the procedure is less invasive and can still provide significant symptom relief [12,13]. Patients which may require intervention of C2 or lower cervical levels are not candidates for minimally invasive approach.
4. Experienced surgeon: MIS for CM1 requires specialized training and expertise. Patients who are considering MIS should seek out a surgeon who has experience with this technique and a proven track record of success [12].
It is important to note that the suitability of MIS for CM1 is determined on a case-by-case basis, and each patient’s individual needs and circumstances must be considered when deciding on the most appropriate treatment approach. Patients with other Chiari malformations, previous decompressive surgery, unstable craniovertebral junction requiring fusion, and cerebrospinal fluid (CSF) abnormalities requiring diversion procedures were excluded from this study.
5. Surgical Procedure1) Pre-operative PlanningA thorough history and physical examination, regular preoperative blood work, and pre-anesthetic risk profile are required prior to surgery. It’s critical that patients and their families comprehend the aims of surgical therapy. The aim is to avoid future neurologic deficit and reduce syrinx growth in the syringomyelia patient. The objective in patients who also experience other symptoms, like headaches, is to lessen the frequency and severity of those that are caused by CM1.
2) Patient Positioning and Skin MarkingAfter induction of general anesthesia, the patient was placed in prone position on a standard operating table, the head was secured in the Mayfield clamp (Integra, Life Sciences, Cincinnati, Ohio, United States). Appropriate padding is used to support the chest and hips, leaving the abdomen free. The neck was placed in flexion and the shoulders were retracted caudally and fixed to the operating table using adhesive tape. The posterior occipital area was prepared and shaved for surgery. A 4 cm horizontal incision was marked immediately under the inion.
3) Skin Incision and Surgical ProcedureScalp and minimal electrocautery were used on skin and subcutaneous tissue to expose the posterior neck muscles. Bilateral paramedian trans muscular dissections were then performed through the trapezius and the semispinalis capitis to reach the posterior arch of C1 and the occipital squama. Following the orientation of the muscle fibers, the dissection was extended caudally in a sagittal plane to expose the posterior border of the foramen magnum, the posterior atlantooccipital membrane and the posterior arch of C1 (Figure 1). Self-retaining retractors were then placed to laterally displace the rectus capitis major. Once the posterior arch of C1 is identified, a subperiosteal dissection of the vertebral artery canal is performed. A delicate elevation and lateral displacement of the artery is performed to achieve a complete resection of the posterior arch up to the lateral mass.
The suboccipital venous plexus, if encountered, was coagulated with bipolar forceps. Soft tissue dissection was followed by a suboccipital craniectomy 3 cm wide accomplished with a high-speed drill and extending all the way down to the foramen magnum to visualize the dura. After osseous decompression, the epidural adhesion band was coagulated and carefully removed (Figure 2).
Opening the dura was performed under the microscope using bilateral vertical durotomies of approximately 3 cm, it was typically performed in a caudal to rostral fashion (Figure 2). We then proceeded to close the dural defect by using one of three techniques of duraplasty; a synthetic duramater substitute (non-autologous graft) sutured with a No 5-0 nylon suture, non-autologous graft with underlay technique and fibrin sealant (Tisseel, Baxter) or autologous cervical fascia graft sutured with No 5-0 nylon suture (Figure 1, 2). Finally, a standard layered closure was done; muscle and subcutaneous tissue with absorbable sutures and the skin with nylon thread.
RESULTSThirty-six subjects underwent posterior fossa decompression through a minimal invasive approach during the study period. Nineteen patients met inclusion criteria and were used for data analysis. Patients had an average age of 34.6 years, and five pediatric patients (26.3%) were included (Table 1).
1. Patient Characteristics and Re-operative SymptomsAll nineteen patients were treated with posterior fossa decompression with a MIS approach for CM1 related symptoms as the main indication for surgery. The average age at surgery was 34.6±17.5 years and we had patients from 5 to 61 years. 89.5% of the patients were women (Table 1).
The chief complaint of the patients was headache (78.9%) and neck pain (57.9%). Tinnitus, vertigo, and dysphagia had a prevalence of 10.5% each (Figure 3). One patient reported nausea and another subject referred visual alterations. Motor weakness was the most common alteration on neurologic examination present in 5 patients. Myelopathy, gait disturbances and sensory changes had a prevalence of 15.8%.
2. Pre-operative ImagingEvery patient taken into surgery was initially studied with brain and cervical MRI and CT scan. MRI was used to confirm diagnosis of CM1 and rule out additional causes of tonsillar displacement. Cervical CT scan was mandatory in preoperative studies to characterize any vascular or osseous anomaly in the craniocervical junction. Tonsillar herniation ranged from 5 to 17 mm, with an average 8.6 ±2.9 mm. Syrinx was present in 68.4% of patients, in which a complete neuroaxis MRI was done to determine the size of the syringomyelia and the presence of associated scoliosis (Figure 4). Four of the patients (21.1%) had scoliosis and were studied with a panoramic x-ray, thoracic and lumbar MRI in search of secondary causes of scoliosis.
3. SurgeryOperative procedures were done in one hospital by two different neurosurgeons using the technique illustrated previously. The average surgical time was 158.2±50.5 minutes, with no significant difference in timing between different specialists (Table 2). Depending on the neurosurgeon in charge of the surgery different duraplasty methods were used. In four patients an autologous graft of cervical fascia was used to close de dura defect, while in the other 15 subjects a non-autologous graft was preferred. In 47.4% of the patients the graft was sutured to the dura with a nylon suture. For the duraplasty of the other 52.6% of patients an underlay technique and fibrin sealant were used. We had no reports of intraoperative complications. The average hospital stay was 3.7 days, with hospitalization ranging from 2 to 13 days (Table 3).
4. Follow-upAll patients underwent a postoperative CT scan the day after surgery. The first follow-up visit was scheduled 15 days after the procedure and thereafter patients were evaluated from 1 up to 74 months. MRI was ordered for all patients at three months (Figure 4); However, for 26.3% the postoperative MRI could not be retrieved and assessed.
5. Post-operative Symptoms and ComplicationsThe most common post-operative complications were associated with dura closure, including 6 patients with pseudomeningocele and one patient with CSF leak (Table 3). The patient with CSF leak developed bacterial meningitis which was treated with antibiotics and had a favorable outcome. Half of the patients with pseudomeningocele had associated chemical meningitis.
We segregated our data to determine the duraplasty method used and compare complications (Table 4). In ten patients non-autologous graft was used and closed with an underlay technique with fibrin sealant. In this group we had our only CSF leak who developed bacterial meningitis, and two pseudomeningocele, one who had associated chemical meningitis. Five subjects had duraplasty with non-autologous graft and nylon suture, three of them developed pseudomeningocele and two had chemical meningitis. Only four patients had autograft for the duraplasty, one of them developed a pseudomeningocele and none had meningitis. We had no hematomas and no mortality associated with this procedure.
Both clinical and radiographic changes were evaluated after surgery. The CCOS was used to evaluate patients. The average CCOS was 14.3±1.8, with 89.5% of patients having a score between 13–16, showing improvement and good outcome. One patient had a score of 8 and was taken for a reintervention which involved a C2-C3 laminectomy and resection of a cervical arachnoidocele. This was performed to widen the posterior fossa decompression. Patients had a post-operative MRI to evaluate complications and changes, unfortunately 5 of these could not be assessed. An improvement in the posterior fossa was evident in 68.4% of patients. The subject who had the CCOS score of 8 had no change in his posterior fossa image and was taken to a second surgery. Of the patients with a pre-operative syrinx, 46.2% had an improvement in the size of the syrinx and 23.1% were unchanged. There was no increase in syrinx in postoperative images. The VSS was used to assess the esthetic component of the incision, with 87% of the patients with barely notable scars.
DISCUSSIONChiari malformation type 1 was originally described by Hans Chiari in 1896 as an “elongation of the tonsils and the medial parts of the inferior lobes of the cerebellum into con-shaped projections which accompany the medulla oblongata into the spinal canal” [14]. A common treatment for symptomatic CM1 is posterior fossa decompression; there is debate around the technical aspects of surgery, however, the outcomes and risks of surgery are well documented [14].
The first Chiari decompression procedure was performed by James Gardner in 1950 by a wide craniectomy to open the 4th ventricle and plug the obex with a piece of muscle [14]. Since then, multiple variations have been incorporated into Chiari surgery. Sub-occipital posterior fossa decompression with atlas laminectomy and an augmentative duraplasty are considered the standard surgical approach for most symptomatic patients [15]. Decompression of the posterior fossa often yields favorable results. According to published studies, symptoms improve in 60% to 100% of patients, and the success rate for resolving syringomyelia is similar [16].
Different minimally invasive surgical techniques have been proposed for posterior fossa decompression of CM1. Caffo et al. [13], reported twenty-six patients with CM1 with and without syringomyelia who underwent a MIS PFD through a 3×3 cm craniectomy with the removal of the most median third of the posterior arch of C1 and duraplasty [17]. A midline skin incision was performed starting 1 cm above the inion to the spinous process of C2; the fascia and muscles were incised and dissected in a subperiosteal fashion until the occipital bone and the posterior arch of C1 were exposed [13]. Signs and symptoms improved in 76.9% of cases [13]. In their experience the rate of complications was 23% including fistula, worsening symptoms, and respiratory impairment [13].
Quillo-Olvera et al. [17], proposed a micro-decompression of the suboccipital bone, posterior arch osteotomy of C1, and duraplasty through a 2 cm midline incision under surgical microscope magnification. When the suboccipital bone was identified, the medial occipital insertion of the semispinalis capitis muscle, rectus capitis posterior minor, and the medial portion of the rectus capitis posterior major muscles were detached on each side [17]. We believe that even though small craniectomies and incisions were used, the need to detach the muscles may have increased postoperative pain and alter spinal biomechanics; variables that were not studied.
Teo et al. [1] reported a MIS technique in which a tube is inserted through the incision under microscopic guidance to expose the foramen magnum and posterior arch of C1. Five patients underwent this technique, and 9 patients underwent open posterior fossa decompression. One MIS patient and 2 patients from open posterior decompression developed CSF leak post-operatively and required repeat surgery for repair [1]. MIS posterior fossa decompression conferred higher rates of post-operative improvement in quality-of-life measures, and lower rates of post-operative complications [1].
We propose a minimally invasive technique using naturally occurring trajectories to complete the standard surgical objectives. Taking advantage of spaces between muscles obviates the need for muscle lesioning and helps improve post-operative pain, without impairing proper visualization and size of decompression. As documented, a smaller than average incision is enough to create an anatomical corridor wide enough to expose all the surgically relevant structures (occipital squama, posterior arch of C1 and the duramater extending from the posterior fossa all the way down to the cervicomedullary junction). Furthermore, conservation of the nuchal ligament attachment plays an important role in avoiding cervical mechanical and radiologic instability [18].
Due to the extensive exposure obtained, our approach creates a dynamic corridor that allows for more invasive modifications to suit each surgeon’s preferences. Without further exposure or dissection, the posterior arch of C1 can be removed, adequate durotomies with duraplasty, arachnoid dissections, and even tonsillectomies can be carried out if necessary. Although the best procedure is still unknown, there is a decent amount of agreement on the size of the occipital boney resection, and it is typically advised to perform a craniectomy that is between 3–4 cm in diameter extending all the way to the foramen magnum [14]. With the use of our MIS strategy, we were able to remove the complete posterior arch of C1 and perform appropriate decompressive craniectomies that extended all the way to the craniocervical junction. Also, to broaden the decompression, we execute bilateral 3 cm long dural apertures with duraplasty and resect the epidural adhesion band at the cervicomedullary junction.
According to the degree of decompression obtained with the durotomy and the direct visualization of the cisterns, we think the decision to coagulate the cerebellar tonsils should be taken intraoperatively. In none of our cases was a tonsillectomy necessary. However, they were visualized with the MIS approach and if necessary, the tip of the tonsils could be coagulated through this surgical corridor, a practice that is common in some institutions [14,19].
Length of stay (LOS) was compared to a meta-analysis reported by Lu et al. [20]. LOS and blood loss ranged from 3.3 to 6.4 days and 47 to 80 mL, respectively. When compared to our study population, our overall LOS was slightly lower with a mean of 3.7±2.42 days. The estimated blood loss in our study was 154 mL, significantly higher than that reported in literature [20]. We acknowledge that there is a discrepancy between our findings and previous research, and we believe this may be related to an overestimation of blood loss due to human error. As we noted, extreme values can have a significant impact on the mean value, especially in small sample sizes. Nonetheless, during the MIS procedure we observed that smaller muscular dissection helped keep the subarachnoid space clear from blood contamination which might diminish the risk of postoperative arachnoid adhesions and aseptic meningitis.
Clinical improvement has been reported to range from 50% to 86% in recent research, but the parameters used to quantify this vary between authors [21,22]. Our outcome measurements were based around three distinct factors: scar development, objective clinical indicators, and pain and associated symptoms. We observed no symptom recurrence after the follow-up. We used the Chicago Chiari Outcome Scale to determine if there was an objective improvement after surgery, evidencing that 89.5% of patients had scores between 13–16, showing a good outcome. Only one of our patients had a low CCOS score and was taken to a reintervention.
We decided to implement the Vancouver Scar Scale to assess the esthetic outcome of the intervention. As far as we are aware, scarring and neck muscle atrophy in individuals receiving posterior fossa decompression for the treatment of CM1 have not been investigated. We believe that a smaller horizontal incision that remains hidden below the inion and less muscle dissection with preservation of muscles and ligaments which are fundamental for spinal biomechanics and stability of the craniocervical junction causing a lower incidence of muscle atrophy and a better esthetic result.
Post-operative MRI demonstrated a reduction in syrinx size in 46.2% of patients. Unfortunately, we had no image control in 30.8% of subjects, which limits our analysis. Literature reports syrinx improvement in around 60% of patients, but this change may take up to 30 months, and may be missed in initial follow up [10]. Though radiological findings are important for outcome analysis, clinical improvement may appear first, and our results are congruent with those described in other studies, demonstrating an appropriate decompression through our minimally invasive technique.
Overall complications associated with posterior fossa decompression for CM1 vary widely through literature and depend on the type of surgery performed and the surgeon’s experience [23]. CSF leak, aseptic meningitis, and pseudomeningocele are the most common complications [6]. Graft complication rates reported in studies range between 18 and 40% [24]. In our population pseudomeningocele was the most frequent complication, occurring in 31.6% of our patients, and disappearing during follow up. 15.8% of the patients had chemical meningitis. Both complications were associated with non-autologous grafts, as reported in the Park-Reeves Syringomyelia Research Consortium study [24]. It is worth mentioning that in the three patients in our cohort that developed chemical meningitis a bovine graft was used. There was no significant difference between complications using nylon suture vs. underlay technique with fibrin sealant.
The limitations of this involve a small sample size, with no follow up MRI in every patient. This is a retrospective study without a control group limiting the interpretation of the findings. However, during the period evaluated, a trend was observed in the improvement of clinical results. Further prospective studies comparing out technique with other common techniques could confirm the benefits of performing this minimally invasive approach.
CONCLUSIONDifferent surgical techniques have been proposed for posterior fossa decompression of CM1. In the present study, we favor a minimally invasive approach to the craniocervical junction to preserve as much as normal anatomy as possible and avoid alterations in spinal biomechanics. The surgical technique that we have described takes advantage of a minimally invasive corridor to decompress the posterior fossa while preserving the posterior tension band with minimal muscle disruption. We believe this approach presents several advantages over traditional midline procedures. However, further investigation of this technique, with a prospective larger sample size and long-term clinical and radiologic follow-up, is necessary.
NOTESEthical statements The study received approval from the appropriate ethical review board before being conducted, which ensures that the research adhered to ethical standards and regulations. In addition, the study obtained informed consent from all patients, which indicates that they were fully aware of the risks and benefits of the procedure and willingly agreed to participate. Fig. 1.Minimally invasive CM1 posterior fossa decompression. (A) 4 cm horizontal skin incision under the inion. (B, C) Bilateral muscular dissection is performed though the trapezius and semispinalis capitis. We continue the dissection caudally after reaching the occipital squama to reveal the foramen magnum’s border, the atlantooccipital membrane, and the posterior arch of C1 (asterisk). (D) Dural graft covering the dural incision. (E) Muscle closure with absorbable suture. Black arrows indicate the position of the head in each image. 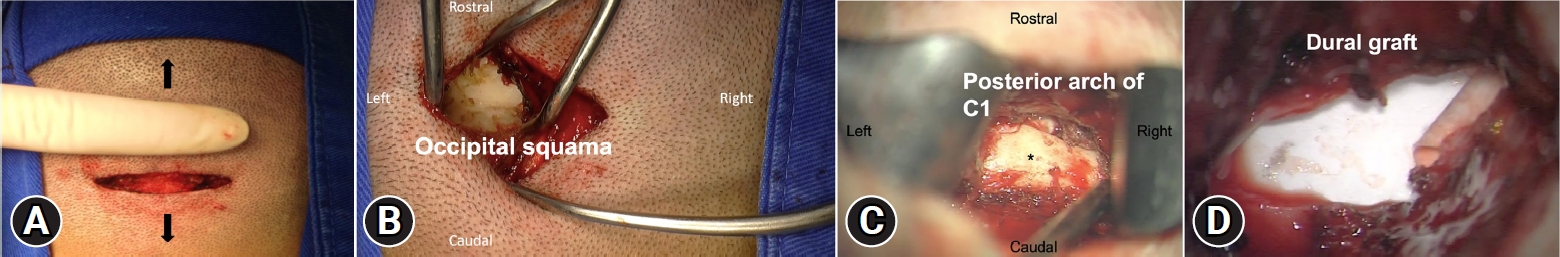
Fig. 2.Surgical technique for CM1 MIS PFD. (A) Skin incision. (B) Muscular dissection is performed bilaterally, here we see the right paramedian trans muscular dissection, through the trapezius and the semispinalis capitis, exposing the occipital squama. Upon reaching the occipital squama we continue the dissection caudally to expose the edge of the foramen magnum, the atlanto occipital membrane and the posterior arch of C1. (C, D) Posterior fossa decompression (craniectomy 3 cm in diameter and resection of posterior arch of C1) with a high-speed drill. After bone resection the epidural adhesion band is coagulated and removed. Bilateral 3 cm durotomies are performed, closure with dural patch and reinforcement with fibrin sealant or nylon suture is done. 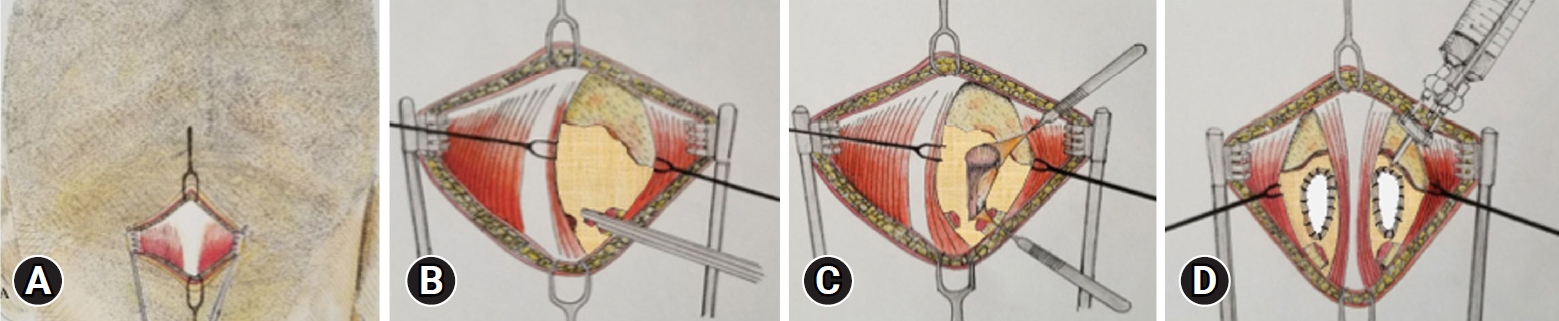
Fig. 4.Comparison of pre-operative (A–C) and post-operative (D–F) images (cervical MRI T2-sequence) of a 48-year-old patient who presented with a history of occipital headache and upper limb weakness that exacerbated with Valsalva maneuvers. The patient underwent minimally invasive posterior fossa decompression without any complications. Pre-operative images: (A) Sagittal section, showing syrinx extending to the level of the vertebral body of C2. There is evidence of descent of cerebellar tonsils 7 mm below the foramen magnum. (B) Axial section at the level of the middle third of the odontoid, showing tonsillar descent. (C) Axial section at the level of the C3 vertebra showing syrinx. Post-operative images: (D) Sagittal section showing resolution of syrinx, C1 posterior arch laminectomy and adequate decompression of the posterior fossa. (E) Axial section, showing laminectomy of the posterior arch of C1. (F) Axial section at the level of the C3 vertebra showing resolution of the syrinx. 
Table 1.Patient characteristics
Table 2.Surgical variables in minimally invasive posterior fossa decompression for Chiari malformation type 1 REFERENCES1. Teo KA, Yang L, Leow R, Lwin S, Kuo JS. Minimally-invasive approach to posterior fossa decompression: initial experience in adult Chiari type 1 malformation patients. J Clin Neurosci 2018;56:90–94.
3. Ciaramitaro P, Massimi L, Bertuccio A, Solari A, Farinotti M, Peretta P, et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: international consensus document. Neurol Sci 2022;43:1327–1342.
4. Sakushima K, Tsuboi S, Yabe I, Hida K, Terae S, Uehara R, et al. Nationwide survey on the epidemiology of syringomyelia in Japan. J Neurol Sci 2012;313:147–152.
5. Brickell KL, Anderson NE, Charleston AJ, Hope JK, Bok AP, Barber PA. Ethnic differences in syringomyelia in New Zealand. J Neurol Neurosurg Psychiatry 2006;77:989–991.
6. Offiah CE, Day E. The craniocervical junction: embryology, anatomy, biomechanics and imaging in blunt trauma. Insights Imaging 2017;8:29–47.
7. Díaz RC, Berbeo ME, Quintero ST, Acevedo JC, Zorro OF, Feo OH. Craniocervical junction diseases treatment with a minimally invasive approach. Coluna/Columna 2014;13:129–132.
8. Aliaga L, Hekman KE, Yassari R, Straus D, Luther G, Chen J, et al. A novel scoring system for assessing Chiari malformation type I treatment outcomes. Neurosurgery 2012;70:656–664.
9. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty 2010;10:e43.
10. Alexander H, Tsering D, Myseros JS, Magge SN, Oluigbo C, Sanchez CE, et al. Management of Chiari I malformations: a paradigm in evolution. Childs Nerv Syst 2019;35:1809–1826.
11. Rocque BG, George TM, Kestle J, Iskandar BJ. Treatment practices for Chiari malformation type I with syringomyelia: results of a survey of the American Society of Pediatric Neurosurgeons. J Neurosurg Pediatr 2011;8:430–437.
12. Kotil K, Ozdogan S, Kayaci S, Duzkalir HG. Long-term outcomes of a new minimally invasive approach in Chiari type 1 and 1.5 malformations: technical note and preliminary results. World Neurosurg 2018;115:407–413.
13. Caffo M, Cardali SM, Caruso G, Fazzari E, Abbritti RV, Barresi V, et al. Minimally invasive posterior fossa decompression with duraplasty in Chiari malformation type I with and without syringomyelia. Surg Neurol Int 2019;10:88.
14. Klekamp J. Surgical treatment of Chiari I malformation--analysis of intraoperative findings, complications, and outcome for 371 foramen magnum decompressions. Neurosurgery 2012;71:365–380.
15. Siasios J, Kapsalaki EZ, Fountas KN. Surgical management of patients with Chiari I malformation. Int J Pediatr 2012;2012:640127.
16. Tubbs RS, Beckman J, Naftel RP, Chern JJ, Wellons JC 3rd, Rozzelle CJ, et al. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation type I. J Neurosurg Pediatr 2011;7:248–256.
17. Quillo-Olvera J, Navarro-Ramírez R, Quillo-Olvera D, Quillo-Reséndiz J, Kim JS. Minimally invasive craniocervical decompression for Chiari 1 malformation: an operative technique. J Neurol Surg A Cent Eur Neurosurg 2019;80:312–317.
18. Sakaura H, Hosono N, Mukai Y, Oshima K, Iwasaki M, Yoshikawa H. Preservation of the nuchal ligament plays an important role in preventing unfavorable radiologic changes after laminoplasty. J Spinal Disord Tech 2008;21:338–343.
19. Won DJ, Nambiar U, Muszynski CA, Epstein FJ. Coagulation of herniated cerebellar tonsils for cerebrospinal fluid pathway restoration. Pediatr Neurosurg 1997;27:272–275.
20. Lu VM, Phan K, Crowley SP, Daniels DJ. The addition of duraplasty to posterior fossa decompression in the surgical treatment of pediatric Chiari malformation type I: a systematic review and meta-analysis of surgical and performance outcomes. J Neurosurg Pediatr 2017;20:439–449.
21. Durham SR, Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr 2008;2:42–49.
22. Litvack ZN, Lindsay RA, Selden NR. Dura splitting decompression for Chiari I malformation in pediatric patients: clinical outcomes, healthcare costs, and resource utilization. Neurosurgery 2013;72:922–928.
23. Batzdorf U. Short-term and long-term complications associated with posterior fossa decompression for chiari malformation. Neurosurg Clin N Am 2023;34:113–117.
24. Yahanda AT, Adelson PD, Akbari SHA, Albert GW, Aldana PR, Alden TD, et al. Dural augmentation approaches and complication rates after posterior fossa decompression for Chiari I malformation and syringomyelia: a Park-Reeves Syringomyelia Research Consortium study. J Neurosurg Pediatr 2021;27:459–468.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||