INTRODUCTION
It has been constantly attempted by surgeons along the entire globe to achieve optimum surgical outcomes with minimal collateral damage in spinal surgeries. This thought formed the core idea behind minimally invasive spine surgery (MISS) which reduces the morbidity of the conventional surgical technique while providing a favorable outcome at par or beyond that of the conventional route. The atlanto-axial joint has a large range of movement, but that movement comes at a cost of increased instability which is frequently encountered in clinical practice. This condition has been well documented and researched with a variety of surgical procedures devoted in providing a substantiated long-term solution [1,2]. Gallie [3] and Brooks, Jenkins [2] described Posterior wiring technique which requires external immobilization and were associated with high complication rates. Then instrumentation such as C1-C2 trans-articular screw (TAS) fixation and screwrod construct (SRC) have revolutionized the management of atlantoaxial instability (AAI). Magerl and Seemann [5] in 1979 introduced The TAS technique as a supplement to Gallie’s fixation which resulted in 98% fusion rates among patients. Biomechanical studies evaluating the efficacy of posterior techniques in resisting pure movements reaffirm the superiority of TAS [7]. The literature is in favour of stand-alone TAS which provides excellent rotational and lateral bending stability but is not as effective at resisting flexion and extension loads. The process can be potentially injurious to the neuro-spinal or neuro-vascular structures owing to their anatomical proximity and probabilities of variations among patients [9]. We refer it as MIS-TAS because of the principles used to minimize surgical trauma by using smaller incision, avoid any damage to interspinous ligament and capsule of C2-C3 joint, use of allograft in place of the conventional autograft for support and by avoiding the use of wires. The technique has a steep learning curve and is associated with unforgivable complications.
The purpose of the present article is to evaluate the learning curve of C1-C2 MIS-TAS based on clinical and surgical parameters and to delineate the challenges encountered during initial cases while providing recommendations to avoid the problems.
MATERIALS AND METHODS
1. Methodology
After taking institutional ethical board committee approval, data of first 84 patients with different etiologies operated by C1-C2 MIS-TAS technique between 2009 and 2014 by a single surgeon at a single institute with a minimum follow-up of 2 years were retrieved (Table 1). All subjects were apprised of the details of the study and written informed consent for their inclusion was obtained. The patients with diagnosed mobility and reducibility of C1-C2 instability with no evidence of basilar invagination were included in the study. Those having irreducible C1-C2 instability, evidence of basilar invagination, articular mass destruction along C1 and/or C2, evidence of kyphosis in the cervicothoracic region were excluded from the study. In all the patients CT scan of occipito-cervical region and CT angiography for evaluation of the course of vertebral artery was done.
These 84 patients were arranged in sequence of their dates of surgery and divided into four quartiles with 21 patients each and each consecutive group serving as a control for prior.
Demographics, Pre- and post-operative clinical parameters (pain scores: VAS; functional disability: ODI and mJOA scores), perioperative parameters (operative time, blood loss, and hospital stay), technical issues (guide wire migration, broken guide wire, dorsal screw burst out and poor screw trajectory), complications (dural tear, neural injury, infection, vertebral artery injury) and fusion rates (according to the Bridwell criteria) were evaluated.
2. Operative Procedure
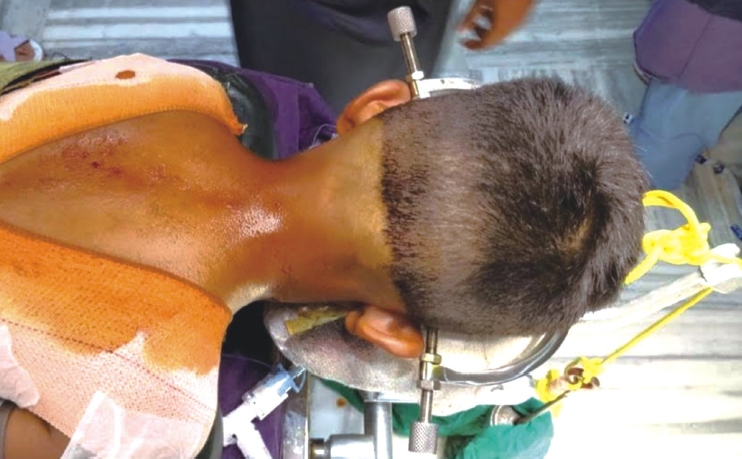
After anesthetic induction, the patient was placed in the prone position and cranium was fixed using a Gardner-Wells tong along with appropriate weight traction (Figure 1).
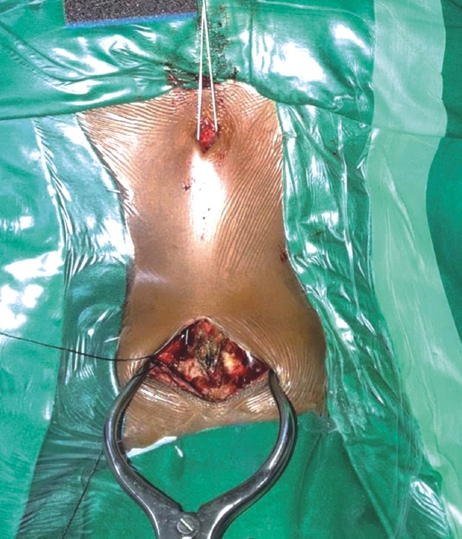
Fluoroscopic imaging was done prior to rigid fixation of Gardner-Wells head holding tong to the table so as to ensure proper alignment and positioning. A C2 centered skin incision was made with an objective to subperiosteally expose the inferior arches of C1 and C2 vertebrae and to not disturb the facet capsule and interspinous ligament of C2 and C3. The C2 pars interarticularis was exposed and clearly defined. Prior to the initiation of drilling, the C1-C2 joint was opened using a Mcdonald or penfield and the joint surface was curetted. The extreme dorsal and medial facets of C1 and C2 articulation line over the inferior aspect of C2 lamina were retrogradely projected to provide a site of entry as well as probable direction. Incision was made at a point located 1cm laterally to the T1-T2 midline region.
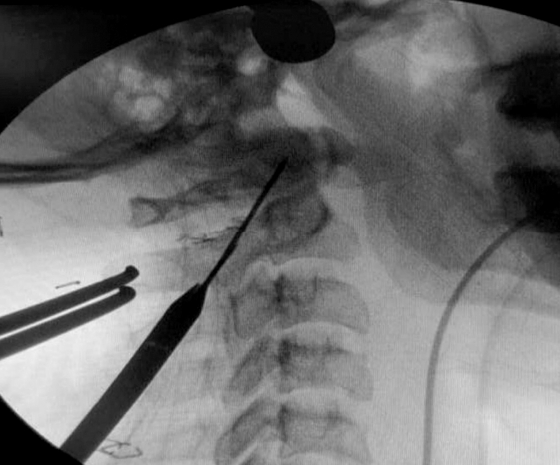
Using a drill guide, once firmly positioned, a high-speed burr was utilized to provide an initial hole/starter hole at the designated entry site at level of C2 to operationalize the trans-articular fixation process (Figure 2). Entry was made 2-3 mm medial from line draw from the medial border of pars, just lateral to the spinous process of C2. The trajectory was determined on the basis of a retrograde projection. The angle made was the line drawn from this point towards the lamina, in the pars inter-articularis through the facet joint of C1-C2 to the lateral mass of C1 till the anterior arch of the C1. Drilling was progressed at the dorsal most and medial most plane to reduce the chances of vascular injury (Figure 3). In previously confirmed high-riding vertebral artery cases, the direction employed was steep as compared to the routine cases. After drilling to the anterior cortex of C1, the hole was tapped and the screw was passed. The procedure was repeated on the contralateral side. Decortication procedure was done on the laminar surface of C2 and arch of C1 by employing the use of a high-speed burr prior to bone graft insertion. The allograft obtained from bone bank was morselized and placed in between the C2 spinous process and posterior arch of the C1 vertebrae in line with the bilateral facet joint.
A single dose of intravenous antibiotic was given preoperatively and post-operatively as a standard protocol and most of the patients were discharged within 48-72 hrs post-surgery. All the patients were advised to wear soft cervical collar and followed up at 10-12 days post-surgery for removal of stitches. Neck exercises started at 3 weeks. Patients were followed up at regular intervals (six weeks, three months, six months, and yearly) and evaluated for clinical, radiological parameters and post-operative complication.
Standard SPSS 20.0 software was used for statistical analysis. The parameters in different quartiles were compared using analysis of variance test. A value of p<0.05 was considered statistically significant.
RESULTS
The mean age of the patients was 36.26±5.78 years (20-78 years) with male to female ratio of 48:36. The patient’s distribution in each quartile was uniform and homogeneous with regards to demographics.
The mean operative time was 113.47±13.13 min(range: 90-180 mins) (q1=122.11±15.05 min, q2=120.88±11.22 min, q3=109.45± 17.15 min, q4=101.15±9.10 min). The mean blood loss was 119.75 ml±14.64 ml (q1=136.88±17.70 ml, q2=128.97±14.22ml, q3=110.12 ±9.88 ml, q4=105.54±16.78 ml). A statistically significant difference was observed between the operative time (p<0.0145) and blood loss (p<0.0001) in second and third quartile. The mean blood loss and operative time in third and fourth quartile was reduced significantly as compared to the first and second quartile due to familiarity, increased skill and confidence in performing the procedure (Table 2).
The mean duration of hospital stay was 5 days (4-7 days) with no significant difference among the different quartiles. VAS(preop, postop), ODI (preop, postop) and mJOA (preop, postop) scores showed a significant improvement from their pre-operative values in the entire study population, but there was no significant difference between the quartiles (Table 3).
There were 3 cases of inadvertent vascular damage (q1=2, q3=1) during the insertion of second screw, but were managed with the help of tamponade and screw insertion with uneventful sequelae. There were 4 incidences of guide wire migration in first quartile which reduced to 2 in second and single in third and fourth quartile. There were 2 incidences of broken guide wires in first quartile which were removed during the surgery. Total 9 times (q1=5, q2=2, q3=1, q4=1) dorsal burst into C2 pars occurred. Significant reduction in dorsal burst of screw in C2 pars was observed between first and the fourth quartile There was no adverse neurological manifestation during or after the procedure in any group (Table 4). In all the cases radiological fusion was seen at final follow-up and the mean duration of fusion was 10.2 months.
1. Case
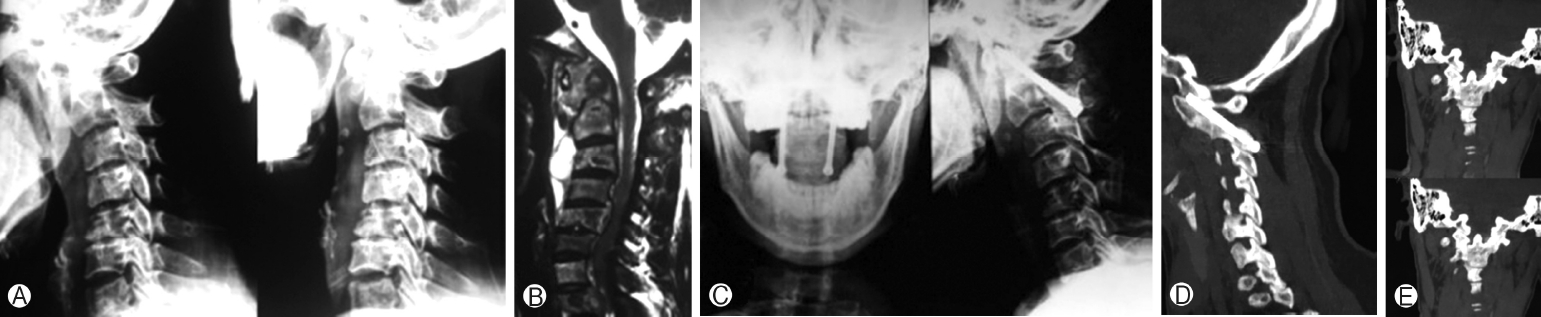
Figure 4. 34-Year-old female with atlantoaxial instability secondary to tuberculosis. (A) Preoperative flexion extension radiograph showing atlantoaxial instability. (B) Preoperative magnetic resonance imaging showing destruction of axis with increase soft tissue mass around C1-C2. (C) Postoperative radiograph showing fusion but not extending completely from C1 to C2. (D) Sagittal view of follow-up CT scan showing C1-C2 union. (E) Coronal view of postoperative CT scan showing intra-articular fusion. CT, computed tomography.
DISCUSSION
Atlantoaxial instability (AAI) is characterized by excessive movement at the junction between the atlas (C1) and axis (C2). The different methods for C1-C2 fixation include posterior methods with interlaminar clamps, wiring techniques, pedicle screw fixation and trans-articular screw fixation. TAS fixation with wiring has advanced in its use in surgical practice owing to the high fusion rates of the instability articulations resulting from a 3-point fixation. We hypothesized that the posterior wiring construct may be non-essential for fusion. The reason we arrived at this hypothesis was because of multiple literature reporting that a sustained excellent outcome is possible despite wire failure. In our previous study we found a fusion rate of 97.5% without the use of wires [10]. Matsumoto et al and Ito et al in their articles mentioned a fusion rate of 95% and 100% respectively despite their cases having confirmation of wire loosening [4,6]. which provide a doubt over the utility of posterior wire usage, nonetheless the efficacy of wiring in providing range mobility remains undisputed. With any procedure with time and experience operative time, blood loss and complication rate decreases. In our study we observed that there was a steady decline in the operative time and blood loss from first to fourth quartile with significant decline from second to third quartile.
Duration of surgery, quantity of blood loss and hospital stay in our study was found to lower than that by other authors [4,11], possibly due to reduction in time required to wire as well as due to the use of an allograft and the risk of infection or other disorders at site of donation in autograft is completely eradicated thus leading to a reduced hospital stay. The incidence of vascular injury in our study was 3.5% compared to other studies with an incidence of up to 8% [8,12]. Pre-operative angiogram was done in all cases to look for vertebral artery anomalies which helped in surgical planning and in reducing vascular injury during the procedure. Also, to keep the guide wire and drill most dorsal and medial is very important. Notably, no instrument failure or additional maneuver or reduction was needed in this procedure due to the anatomic reduction of the articulation as well as availability of large amount of allograft. Incidence of guide wire migration has been seen declining with time in our study. Anterior migration of guide wire could lead to hypoglossal nerve injury which was not seen in any of the cases in our study. There were two incidences (both in first quartile) of broken guide wires in the study which were successfully removed without any sequalae. To prevent breakage of guide wire same trajectory of guide wire has to be followed while tapping and drilling and also to grasp the wire while putting the screw. Common difficulties faced by new learners mastering MIS-TAS technique include improper positioning, inadequate reduction of C1-C2, guide wire migration, improper trajectory, C2 pars burst, vascular and neurological injuries. Therefore, there is a need to understand this technique for the benefit of new learners and clinical spine fellows, to avoid repeated errors faced during initial cases. The asymptote of any procedure is determined by the perioperative parameters (operative time, intraoperative blood loss), procedure effectiveness through the clinical parameters and complications. The challenges in MIS-TAS is its steep learning curve. Atlanto-axial joint is a complex joint with anatomical variations because of this for a posterior C1-C2 TAS proper radiographic anatomic planning is must.
The advantages associated with this approach are all the advantages associated with minimally invasive spine surgery (MISS) like less duration of surgery, less muscle stripping, less blood loss, shorter hospital stay, lower complications and early mobilization. Along with these, avoidance of C1-C2 wiring saves time and prevent from all the wiring related complications, and use of allograft reduces donor site morbidity. We have seen 100% fusion rates without any implant related delayed complications however long term studies are needed to determine that. The disadvantages are a steep learning curve and the limited indications like reducible atlanto-axial instability. To reduce the steep learning curve associated with MIS-TAS the recommendations are: In depth pre-operative assessment of patient and Extensive radiological imaging to ascertain anatomical variations, judicious use of Intra-operative fluoroscopy, using proper fixation and alignment for optimal positioning, use of models or simulators prior to actual surgery for skill enhancement, Practice sessions on cadavers/models, Designing effective mentoring programs.
The limitations in our study was the retrospective nature, small sample size and lack of surgeon diversity. There was no way to assess variation in learning pattern among different surgeons and not all individuals are equally proficient in adapting to newer patterns. The sample population was homogenous and their etiology was varied, however a larger sample size with a broader base and wider demographics would have been a more appropriate setting for definitive learning pattern recognition.
CONCLUSION
C1-C2 MIS-TAS is a very safe and effective means of treating reducible atlanto-axial instability with good clinical and radiological outcomes. Pre-operative planning, detailed radiological evaluation, practice on cadavers/bone-saw models and by following the mentioned recommendations the learning curve of C1-C2 MIS-TAS can be reduced.