AbstractObjective This study investigated the clinical and radiological outcomes of lumbar spinous process-splitting laminectomy (LSPSL) performed to treat lumbar spinal stenosis at a single institution in Korea.
Methods A retrospective review was conducted of patients who underwent LSPSL for lumbar spinal stenosis between June 2020 and February 2022, with a minimum 1-year follow-up. Clinical outcomes were assessed using the visual analogue scale (VAS), Oswestry Disability Index (ODI), European quality of life - 5 dimensions - 5 levels (EQ-5D-5L), European quality of life VAS (EQ-VAS), and modified MacNab criteria. One year after surgery, radiological outcomes were evaluated through computed tomography scan to assess the spinolaminar bone union rate and patterns.
Results Out of 38 patients, data from 30 patients (male:female=17:13) and 36 surgical levels were analyzed. The mean age was 67 years (range, 46–88 years). The preoperative mean leg VAS score and ODI significantly decreased at the 1-year postoperative follow-up (leg VAS, 6.6–3.8; p=0.001; ODI, 19.3–10.9, p=0.006). The EQ-5D-5L index and EQ-VAS also significantly improved (0.52–0.77, p<0.001; 50.8–67.1, p=0.018; respectively). Using the modified MacNab criteria, the study reported excellent and good outcomes in 80% of patients at the 1-year follow-up, with no serious complications observed. The overall spinolaminar union rate was 77.8% (complete union, 58.3%; partial union 19.4%).
INTRODUCTIONLumbar spinal stenosis is characterized by the narrowing of the lumbar spinal canal, which can compress the nerve roots. This condition can cause various symptoms including sensory changes, pain in the back and legs, neurogenic claudication, and even motor weakness of the legs. Initially, conservative managements options such as lifestyle modification, medication, physiotherapy and spinal injections are considered [1]. However, if conservative treatments fail to provide relief, surgery to decompress the spinal canal is recommended [1,2].
Conventional laminectomy is a surgical procedure to treat lumbar spinal stenosis by removing the entire lamina and, situationally, a part of facet joints or ligamentum flavum [3]. While conventional laminectomy can effectively relieve spinal stenosis, this technique is associated with several disadvantages. The removal of the spinous process and the detachment of paraspinal muscles pose potential risks for postoperative spinal instability which can result in persistent back pain and additional spinal deformity [4-7].
Several surgical techniques have been developed to overcome the shortcomings of conventional laminectomy. Among them, lumbar spinous-process splitting laminectomy (LSPSL) was first introduced by Watanabe et al. [6,7] and modified by Nomura et al. [8]. This procedure preserves the integrity of spinous processes and maintains the attachment of the paravertebral muscle insertion. By preserving posterior supporting structures, LSPSL minimizes tissue disruption and postoperative morbidities [9].
LSPSL has demonstrated fair clinical outcomes, but not much data regarding the clinical and radiological outcomes of this approach is available in Korea. This study aims to analyze 1-year clinical and radiological outcomes of patients who underwent LSPSL surgery for lumbar spinal stenosis.
MATERIALS AND METHODSBetween June 2020 and February 2022, patients who received LSPSL surgery for degenerative lumbar spinal stenosis by a single surgeon (SL) at Samsung Medical Center were retrospectively reviewed. Institutional Review Board (IRB) approval was obtained for this study (IRB No. 2023-06-060) and informed consent was waived due to its retrospective nature.
We included patients who had a minimum of 1-year follower-up. Patients with missing medical records and radiographic images during the follow-up period, and those who underwent LSPSL for conditions other than degenerative spinal stenoses, such as herniated intervertebral disc, epidural abscess, and intradural tumors, were excluded. Patients with radiographic instability at the index level or more than grade 2 spondylolisthesis were also excluded. Basic demographic data, the level of operation, postoperative hospital stay, operation time, estimated blood loss during surgery, and follow-up period were collected by reviewing the patient’s medical records. Preoperative evaluation included anteriorposterior, lateral, flexion and extension simple radiographs, computed tomography (CT) and magnetic resonance imaging (MRI) of the lumbar spine for every patient.
During the study period, 55 patients underwent the LSPSL procedure. Out of these, 10 patients received LSPSL for a herniated lumbar intervertebral disc, and 1 patient underwent the procedure for a tumor. As a result, these 11 patients were excluded from the study. Additionally, 14 patients were excluded due to missing medical records, radiographic images and the necessary follow-up. Consequently, 30 patients were included in this study.
1. Surgical Procedures: Lumbar Spinous Process Splitting Laminectomy
Figure 1 illustrates the surgical procedure of the lumbar spinous process splitting laminectomy. All surgeries were performed under general anesthesia. Patients were positioned prone, and we primarily utilized the Wilson frame to flex the patients’ lumbar spine, thereby widening the surgical corridor. Confirming the location of the index spinous process by a simple lateral radiograph is the first step (Figure 1A). A midline skin incision is then made (Figure 1B), followed by splitting the index spinous process (Figure 1C, D). For this splitting procedure, we predominantly used an ultrasonic bone scalpel (Bonescalpel, Misonix, New York, NY, USA), although a small-sized burr or a sagittal saw could be used with similar efficacy. After splitting the spinous process, its base is fractured from the lamina using a straight osteotome (Figure 1E). By spreading the floating, split spinous process laterally, both the left and right sides of the index lamina and interlaminar space are exposed (Figure 1F). Following sufficient decompression through partial laminectomy and flavectomy (Figure 1F), the split spinous process is reapproximated. The subcutaneous layer and the skin are sutured layer by layer to close the wound (Figure 1G).
2. Outcome AssessmentsBack and leg pain scores were evaluated using the visual analogue scale (VAS) for the clinical assessment. The impact of the condition on daily functioning was assessed using the Oswestry Disability Index (ODI). The patients’ overall quality of life was measured using the European quality of life - 5 dimensions - 5 levels (EQ-5D-5L) self-rating questionnaire, and the European quality of life VAS (EQ-VAS). These assessments were conducted at the preoperative stage and at the postoperative 3-, 6-, and 12-month follow-up periods.
To calculate the EQ-5D-5L index, the Korean value set and the equation proposed by Kim et al. [10] were used. Furthermore, patient satisfaction and functional improvement after surgery were evaluated using the modified MacNab criteria.
Similar to the clinical assessment, radiological assessments were conducted at the postoperative 3-, 6-, and 12-month follow-up periods. Lumbar spine simple radiographs were evaluated during each of these postoperative periods to observe any changes in the spinal alignment of the lumbar spine. Additionally, at the 12-month follow-up, a CT scan with 2-mm thickness images was obtained. These images were used to assess the bony union rate and pattern between the lamina and the spinous process. We followed the fusion criteria and union pattern described by Wi et al. [11] (Figure 2). The union rate and pattern were further analyzed by dividing it into subgroups based on the number of decompressed levels.
3. Statistical AnalysisIn this study, most demographic and radiographic data were presented in descriptive statistics. The postoperative values of each clinical assessment were compared with the preoperative values using a t-test. When the distribution of a variable did not follow a normal distribution, the Mann-Whitney U-test was used. The significance level was set at p<0.05. All analyses were performed using IBM SPSS Statistics ver. 27.0 (IBM Co., Armonk, NY, USA).
RESULTSA total of 30 patients who received LSPSL surgery for degenerative lumbar spinal stenosis were included in the final analysis. The patients’ demographic data are summarized in Table 1. The mean age was 67 years (range, 46–88 years). There were 17 men and 13 women. Twenty-four patients underwent surgery for single level, 5 for 2 levels, and 1 for 3 levels. The mean postoperative hospital stay was 5.2±1.6 days (range, 2–10 days). The mean follow-up period was 14.0±3.2 months (range, 12–25 months).
1. Clinical AssessmentsThe clinical outcomes are summarized in Table 2 and Figure 3. The mean VAS scores for leg pain, and ODI showed a statistically significant decrease after the operation, and remained consistent throughout the 1-year follow-up period (leg VAS, 6.6–3.8, p=0.001; ODI, 19.3–10.9, p=0.006). On the other hand, the mean VAS score for back pain showed a significant decrease at the postoperative 3-month follow-up (5.3–3.4, p=0.02), but lost its statistical significance in the later follow-ups. The EQ-5D-5L indexes also showed statistically significant improvement after the surgery throughout the 1-year follow-up (0.52–0.77, p<0.001). In addition, the EQ-VAS scores were significantly improved in the postoperative 3- and 12-month follow-ups (50.8–69.8, p=0.004; 50.8–67.1, p=0.018, respectively). According to the modified MacNab criteria, approximately 97% of the patients showed better than fair clinical outcomes at the postoperative 1-year follow-up (Figure 4).
2. Radiographic AssessmentsBecause CT scans of one patient who underwent 1-level LSPSL surgery for lumbar spinal stenosis were missing at the postoperative 1-year follow-up, 36 operated laminae were evaluated for the spinolamina fusion (Table 3). The postoperative 1-year follow-up CT scans showed a gross fusion rate of 77.8% (comprising 58.3% complete spinolamina union and 19.4% partial union). On the other hand, 22.2% of the operated laminae failed to achieve fusion. Among patients who underwent surgery for 1 or 2 levels, nearly 80% achieved fusion. In one patient who underwent 3-level decompression, only 1 level showed partial union, while the other 2 levels remained nonunion at the 1-year follow-up. However, owing to the lack of large patient samples, whether this finding had any significant difference could not be validated. Regardless of the state of fusion, none of the patients showed any changes in spinal alignment, such as an aggravation of lumbar kyphosis during the follow-up period.
3. Impact of Fusion on Clinical OutcomesWe classified patients into 2 groups based on their radiological outcomes: complete union, partial union versus nonunion. We then analyzed whether these groups had significant differences in clinical outcomes at postoperative 12 months. Three patients who underwent surgery involving 2 or 3 levels and had a mixture of complete union, partial union and nonunion outcomes at each level were excluded. The group categorized as fusion and partial union included 22 patients, whereas the nonunion group comprised 4 patients. There were no statistically significant differences among all of the clinical parameters: VAS for back pain (p=0.24), VAS for leg pain (p=0.71), ODI (p=0.32), EQ-5D-5L indexes (p=0.09), EQ-VAS (p=0.44).
4. Postoperative ComplicationsThere were no serious complications related to the surgery. One patient who complained of grade 4 right ankle weakness and persistent pain 2 weeks after the surgery. A follow-up lumbar spine MRI revealed an epidural hematoma at the operation site. Given that the patient had a low platelet count (41 K) due to hepatocellular carcinoma and liver cirrhosis linked to chronic hepatitis B, we opted for conservative management. This encompassed medication and physiotherapy. Three months later, the patient's symptoms had alleviated, and the motor power in his right ankle had been restored, all without additional treatments or subsequent complications.
DISCUSSION1. Clinical and Radiographic OutcomesAfter LSPSL surgery, approximately 97% of the patients reported better than fair clinical outcomes at the postoperative 1-year follow-up according to the modified MacNab criteria. VAS for leg pain, ODI, EQ-5D-5L indexes, and EQ-VAS showed statistically significant improvements at the last follow-up. VAS for back pain showed statistically significant improvements at the postoperative 3-month follow-up, but lost its significance in the later follow-ups. However, a trend of improvement was noted during the follow-up period. Our results are broadly consistent with the reports in the literature. Cho et al. [12] reported that lower postoperative VAS scores were observed in the LSPSL group compared to the conventional laminectomy group. The muscle-sparing nature of LSPSL showed more favorable outcome, which were consistently observed at the 1-year follow-up. Some authors analyzed the recovery rate of the Japanese Orthopedic Association (JOA) score for clinical assessments [6,12]. JOA score in the LSPSL group was better but not all results met statistically significant results. Similar to our findings, Lee and Srikantha [13] reported a rate of 95% for better than fair clinical outcomes. They explained that the lower proportion of “excellent” outcomes in the elderly set of patients could be attributed to several factors beyond the direct outcome of surgery. Based on these results, we could conclude that the overall clinical outcome of LSPSL surgery at the postoperative 1 year was favorable.
Determining whether the split spinous process and the lamina will recover structurally is important. The gross union was observed in most cases (77.8%). Complete restoration of the spinolaminar structure was observed in 56.5% of single-level surgery cases and 58.3% of the overall levels in the current study. This rate was higher than the original technique by Watanabe et al. [6] (32.9%) but somewhat lower than Nomura et al.’s technique [8] (82.7%). Wi et al. [11] reported a higher fusion rate in the partial spinous splitting group than in the complete spinous splitting group. Because our surgical technique incorporated Watanabe’s total splitting method, this may be contributed to a lower rate of complete union compared to the results reported by Nomura et al. [8]. Because our study was a preliminary study with a small patient population, further research on a large scale is needed to investigate the higher fusion rate associated with partial splitting procedures.
In addition, it is important to determine whether spinolamina fusion affects patient clinical outcomes. Wi et al. [11] reported that no significant differences in the clinical results between patients who obtained complete restoration of the spinolaminar structure and those who obtained partial union or nonunion. Nomura et al. [8] reported that no direct evidence indicated that spinous process floating was associated with unfavorable clinical outcomes. Likewise, our study showed that the spinolaminar structure restoration did not show statistically significant differences in the clinical outcomes. However, by excluding patients with mixed results from multiple levels, there were insufficient patient numbers (4 cases) in the nonunion group. In addition, we have grouped complete and partial unions into 1 category, but it is unclear if they are equivalent. Therefore, further large-scale studies are needed to provide additional evidence in the future.
2. Advantages of Lumbar Spinous Process-Splitting LaminectomyBecause the conventional laminectomy technique utilizes extensive detachment of the paraspinal muscles, back muscle atrophy, chronic back pain, and even spinal instability can be occurred [14-22]. Several studies have conducted quantitative analyses of the paravertebral muscles using T2-weighted images before and after surgery [6,23,24]. Kanbara et al. [25] reported that in a 1-year follow-up, paravertebral muscle atrophy was lesser in the LSPSL group (7.8%) compared to the conventional laminectomy group (22.2%). In this study, the minimally invasive nature of LSPSL, which preserves the back muscles, is believed to have contributed to favorable postoperative clinical outcomes.
Spinal instability after conventional laminectomy has been reported as a major complication [1,26-28]. It has been reported that preserving the structural integrity of the facet joint is beneficial in preventing vertebral slippage after surgery [28-30]. Compared to the LSPSL, midline structures disturb the access to the lateral recesses in bilateral laminotomy [2,6]. LSPSL offers symmetrical surgical visualization of the lateral recesses, and the risk of postoperative spinal instability resulting from excessive facetectomy can be minimized. Nomura et al. [8] also reported LSPSL did not accelerate postoperative slippage or instability of the vertebral body, which is well correlated with our study results.
Despite the benefits of the LSPLS [2], the LSPSL for lumbar spinal stenosis is less popular and less frequently performed compared to the unilateral laminotomy bilateral decompression. We conjecture that this is likely because surgeons are unsure of the benefits of LSPSL surgeries and find this technique cumbersome. In our opinion, the advantages of LSPSL surgery are as follows. First, the LSPSL technique is relatively easy to acquire. As Nomura et al. [8] pointed out, we experienced that this technique did not require a special learning curve. Compared to conventional laminectomy, the LSPSL technique involved a smaller incision while the surgical view was familiar and wide, providing more favorable clinical and radiological results. Second, in cases with severe facet hypertrophy, ipsilateral lateral recess, foraminal visualization, and decompression sometimes require a larger facet resection of the ipsilateral side, predisposing the spine to instability. Because LSPSL provides symmetrical surgical corridors from the midline, such risk could be lowered regardless of the degree of facet hypertrophy. Lastly, handling unexpected surgical complications is much easier in LSPSL. Especially when a dural tear occurs which is one of the common complications during minimally invasive spinal surgeries, primary repair is possible without additional exposure in most cases. Even if a surgeon did not start lumbar decompression surgery with the LSPLS technique, it is worth considering as a salvage technique in a complicated event.
3. Postoperative ComplicationsIn our study, involving 30 patients undergoing surgery for 37 surgical levels, a self-limiting postoperative hematoma was observed in one case where the patient underwent surgery on a single level. The case of the patient occurred in the later part of the study. Postoperative hematoma has been reported to have a complication rate of 0.8% to 1.4% when conventional laminectomy is performed [21,31]. There were no complications such as wound dehiscence or dura tear leading to cerebrospinal fluid leakage, which are commonly observed. Considering that the overall complication rate after a typical lumbar laminectomy ranges from 2.5% to 7%, the postoperative complications observed in our study are considered acceptable [32].
4. LimitationsThis is a retrospective, single-arm study without a control group. Therefore, it has inherent limitations owing to its study design. Also, the small study population limited the overall credibility of the study results. Furthermore, this is a preliminary result of a single center for only 1-year follow-up period. For a proper evaluation of LSPLS outcomes in treating lumbar spinal stenosis, further studies with better design and a large number of patients should be required.
CONCLUSIONWe found that LSPSL provides favorable clinical outcomes and an acceptable posterior bony structure restoration rate, making it a feasible treatment option for lumbar spinal stenosis. We believe that LSPSL is one of the promising minimally invasive decompressive surgery for treating lumbar spinal stenosis. Therefore, future research with a large number of patients and long-term follow-up is required to validate this promising procedure.
ACKNOWLEDGEMENTSThe authors would like to thank Da Hyeun Lee, an audiovisual engineer at Samsung Medical Center Information & Medical Services, for designing Figure 1 for this work.
Figure 1.Illustration of the lumbar spinous process splitting laminectomy procedure. (A) An intraoperative simple lateral radiograph is used to identify the location of the index spinous process. (B) A midline skin incision is made over the confirmed location of the index spinous process. (C, D) The index spinous process is split using an ultrasonic bone scalpel. (E) The spinous process base is fractured from the lamina using a straight osteotome. (F) Split, floating spinous processes are spread laterally to secure the surgical corridor and decompress the index level of stenosis. (G) After sufficient decompression, the operation wound is closed by reapproximating the split spinous processes and suturing subcutaneous and skin layers. 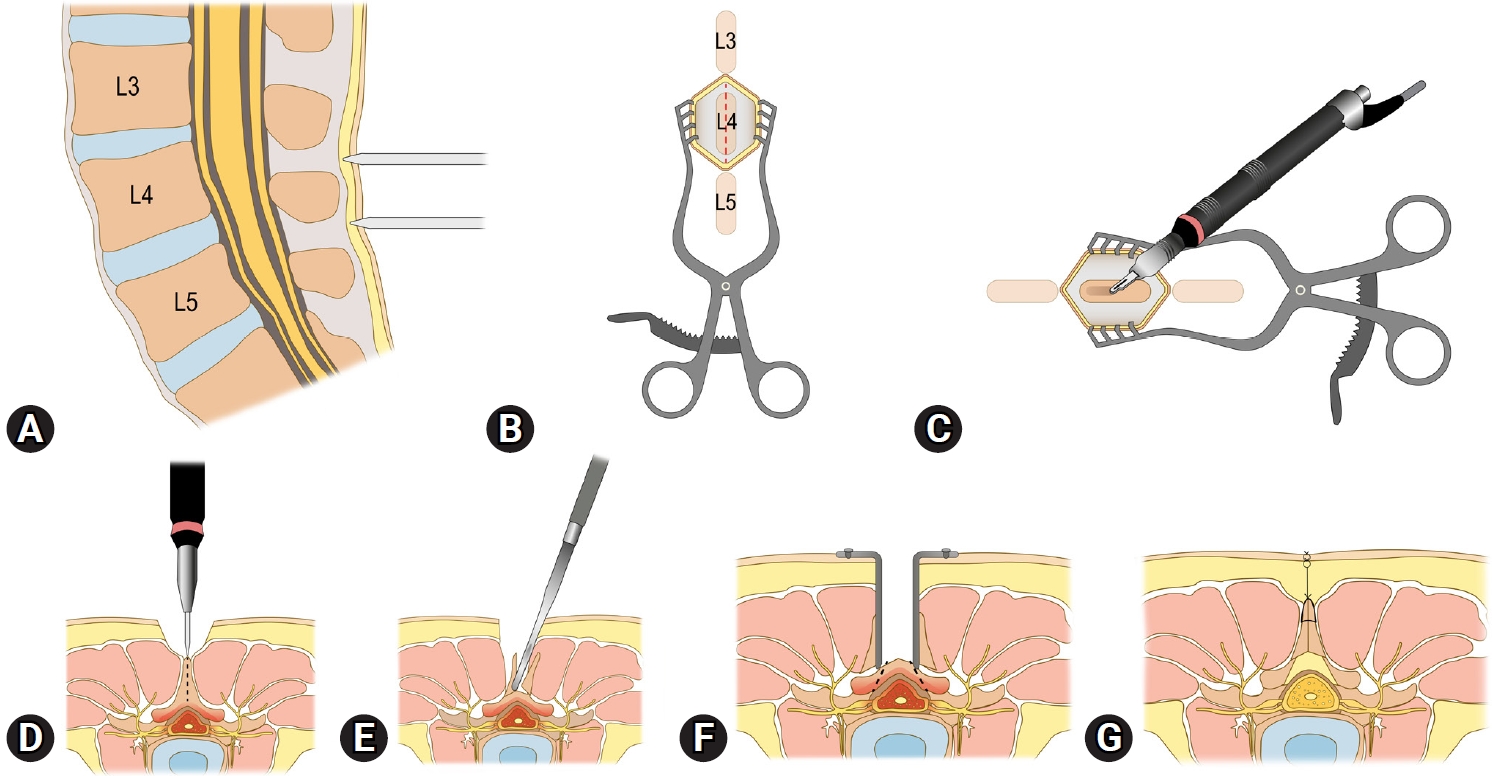
Figure 2.Sagittal (left) and axial (right) computed tomography images taken at the postoperative 1-year follow-up showing (A) complete union between the split spinous processes and at the spinolaminar junction, (B) partial union (i.e., floated or one-side union) of the spinous process, (C) nonunion between the split spinous process and at the spinolaminar junction. The union and nonunion sites are marked with white arrows. 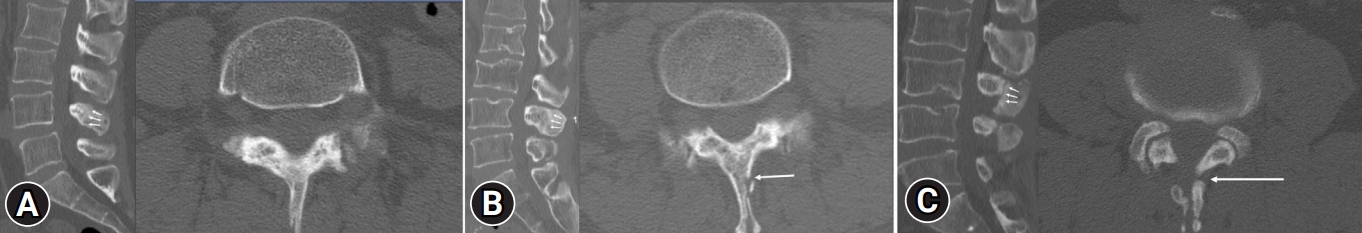
Figure 3.Preoperative and postoperative comparisons of clinical outcomes. (A) Back pain visual analogue scale (VAS) score, (B) leg pain VAS score, (C) Oswestry Disability Index (ODI), (D) European quality of life - 5 dimensions - 5 levels index (EQ-5D-5L), (E) European quality of life VAS (EQ-VAS). 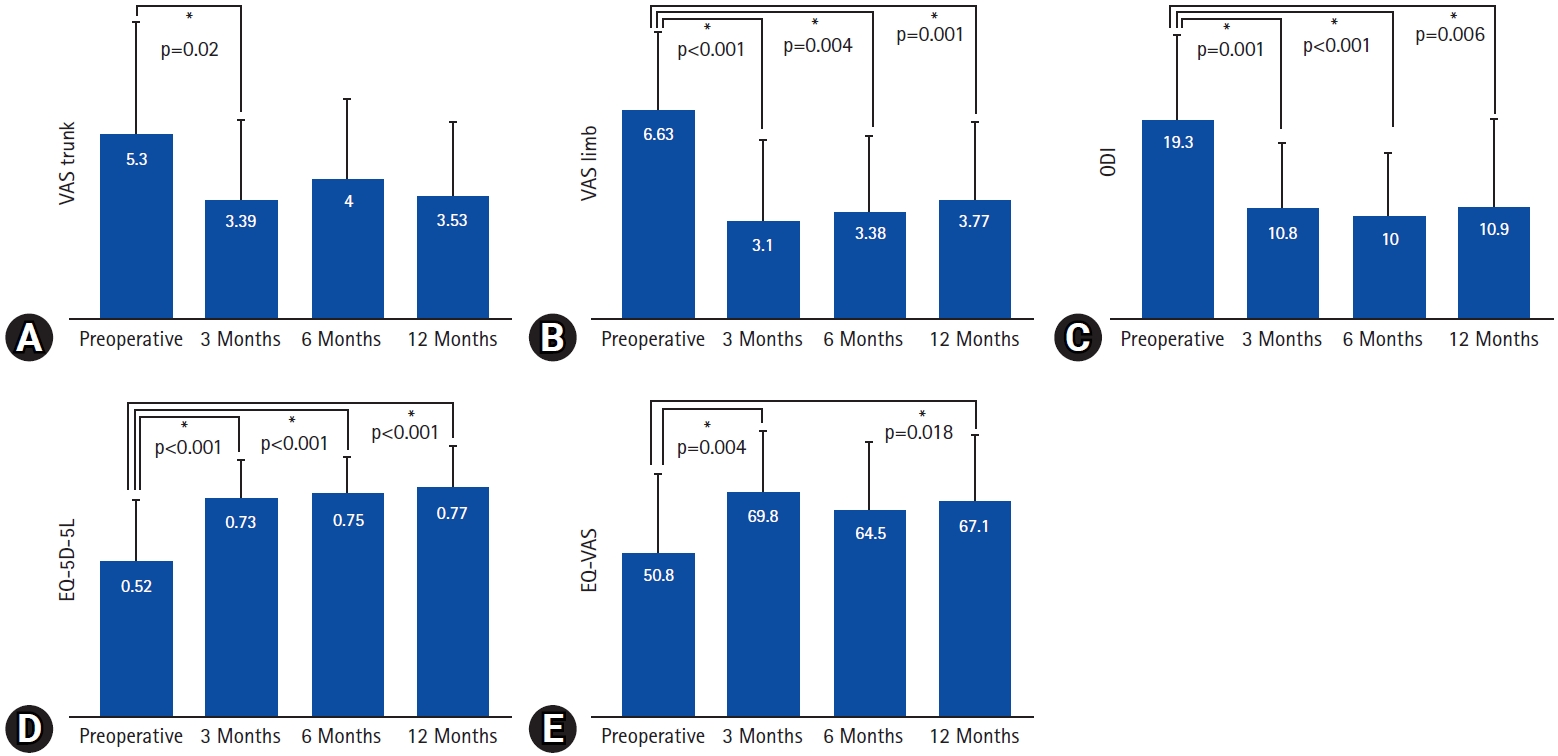
Figure 4.Clinical outcomes at the postoperative 1-year follow-up using the modified MacNab criteria. 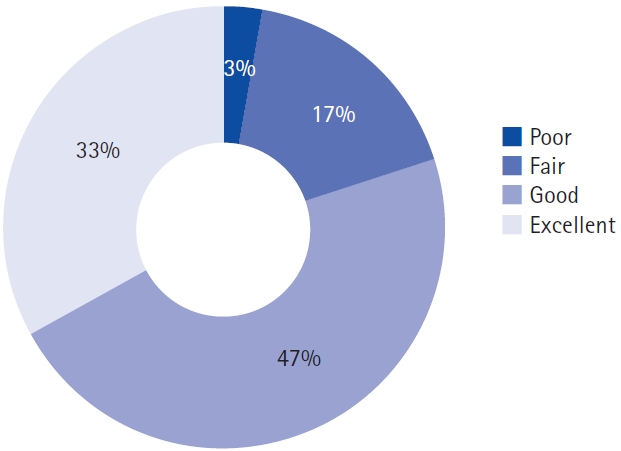
Table 1.Patient demographics (n=30) Table 2.Clinical outcomes (n=30) Table 3.Spinolamina union rates at postoperative 1-year follow-up (n=36)*
Values are presented as number (%). *The computed tomography scan of 1 patient who underwent 1-level lumbar spinous process-splitting laminectomy surgery for lumbar spinal stenosis at the postoperative 1-year follow-up was missing. As a result, 36 laminae were used in the analysis, 1 less than the 37 laminae that should have been analyzed. REFERENCES1. Sengupta DK, Herkowitz HN. Lumbar spinal stenosis: treatment strategies and indications for surgery. Orthop Clin North Am 2003;34:281–95.
2. Overdevest GM, Jacobs W, Vleggeert-Lankamp C, Thome C, Gunzburg R, Peul W. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Cochrane Database Syst Rev 2015:(3):CD010036.
3. Zhang Y, Wei FL, Liu ZX, Zhou CP, Du MR, Quan J, et al. Comparison of posterior decompression techniques and conventional laminectomy for lumbar spinal stenosis. Front Surg 2022;9:997973.
4. Johnsson KE, Willner S, Johnsson K. Postoperative instability after decompression for lumbar spinal stenosis. Spine (Phila Pa 1976) 1986;11:107–10.
5. Katz JN, Lipson SJ, Chang LC, Levine SA, Fossel AH, Liang MH. Seven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosis. Spine (Phila Pa 1976) 1996;21:92–8.
6. Watanabe K, Hosoya T, Shiraishi T, Matsumoto M, Chiba K, Toyama Y. Lumbar spinous process-splitting laminectomy for lumbar canal stenosis. Technical note. J Neurosurg Spine 2005;3:405–8.
7. Watanabe K, Matsumoto M, Ikegami T, Nishiwaki Y, Tsuji T, Ishii K, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine 2011;14:51–8.
8. Nomura H, Yanagisawa Y, Arima J, Oga M. Clinical outcome of microscopic lumbar spinous process-splitting laminectomy: clinical article. J Neurosurg Spine 2014;21:187–94.
9. Uehara M, Takahashi J, Hashidate H, Mukaiyama K, Kuraishi S, Shimizu M, et al. Comparison of spinous process-splitting laminectomy versus conventional laminectomy for lumbar spinal stenosis. Asian Spine J 2014;8:768–76.
10. Kim SH, Ahn J, Ock M, Shin S, Park J, Luo N, et al. The EQ-5D-5L valuation study in Korea. Qual Life Res 2016;25:1845–52.
11. Wi SM, Lee HJ, Chang SY, Kwon OH, Lee CK, Chang BS, et al. Restoration of the spinous process following muscle-preserving posterior lumbar decompression via sagittal splitting of the spinous process. Clin Orthop Surg 2019;11:95–102.
12. Cho DY, Lin HL, Lee WY, Lee HC. Split-spinous process laminotomy and discectomy for degenerative lumbar spinal stenosis: a preliminary report. J Neurosurg Spine 2007;6:229–39.
13. Lee S, Srikantha U. Spinous process splitting laminectomy: clinical outcome and radiological analysis of extent of decompression. Int J Spine Surg 2015;9:20.
14. Kawaguchi Y, Matsui H, Gejo R, Tsuji H. Preventive measures of back muscle injury after posterior lumbar spine surgery in rats. Spine (Phila Pa 1976) 1998;23:2282–7; discussion 2288.
15. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 1: Histologic and histochemical analyses in rats. Spine (Phila Pa 1976) 1994;19:2590–7.
16. Kawaguchi Y, Yabuki S, Styf J, Olmarker K, Rydevik B, Matsui H, et al. Back muscle injury after posterior lumbar spine surgery. Topographic evaluation of intramuscular pressure and blood flow in the porcine back muscle during surgery. Spine (Phila Pa 1976) 1996;21:2683–8.
17. Mariconda M, Zanforlino G, Celestino GA, Brancaleone S, Fava R, Milano C. Factors influencing the outcome of degenerative lumbar spinal stenosis. J Spinal Disord 2000;13:131–7.
18. Radu AS, Menkes CJ. Update on lumbar spinal stenosis. Retrospective study of 62 patients and review of the literature. Rev Rhum Engl Ed 1998;65:337–45.
19. See DH, Kraft GH. Electromyography in paraspinal muscles following surgery for root compression. Arch Phys Med Rehabil 1975;56:80–3.
20. Sihvonen T, Herno A, Paljarvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine (Phila Pa 1976) 1993;18:575–81.
21. Silvers HR, Lewis PJ, Asch HL. Decompressive lumbar laminectomy for spinal stenosis. J Neurosurg 1993;78:695–701.
22. Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br 1954;36-B:230–7.
23. Hyun SJ, Kim YB, Kim YS, Park SW, Nam TK, Hong HJ, et al. Postoperative changes in paraspinal muscle volume: comparison between paramedian interfascial and midline approaches for lumbar fusion. J Korean Med Sci 2007;22:646–51.
24. Lee JC, Cha JG, Kim Y, Kim YI, Shin BJ. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine (Phila Pa 1976) 2008;33:318–25.
25. Kanbara S, Yukawa Y, Ito K, Machino M, Kato F. Surgical outcomes of modified lumbar spinous process-splitting laminectomy for lumbar spinal stenosis. J Neurosurg Spine 2015;22:353–7.
26. Iguchi T, Kanemura A, Kasahara K, Sato K, Kurihara A, Yoshiya S, et al. Lumbar instability and clinical symptoms: which is the more critical factor for symptoms: sagittal translation or segment angulation? J Spinal Disord Tech 2004;17:284–90.
27. Iguchi T, Kurihara A, Nakayama J, Sato K, Kurosaka M, Yamasaki K. Minimum 10-year outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine (Phila Pa 1976) 2000;25:1754–9.
28. Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine (Phila Pa 1976) 2005;30(6 Suppl):S71–81.
29. Herron LD, Trippi AC. L4-5 degenerative spondylolisthesis. The results of treatment by decompressive laminectomy without fusion. Spine (Phila Pa 1976) 1989;14:534–8.
30. Yagi M, Okada E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine 2009;10:293–9.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||