AbstractObjective Posterior full-endoscopic cervical foraminotomy/discectomy (PECF) is used to treat medically intractable cervical radiculopathy. PECF has many potential advantages; however, despite its minimally invasive nature, complications of PECF are possible, including hemorrhage, infection, injury to neural tissue, damage to the facet joint and musculature, loss of cervical lordosis, and subsequent progression to cervical kyphosis. We examined complications following PECF and reviewed the relevant literature.
Methods We retrospectively reviewed 101 patients who underwent PECF for either disc herniation (DH, 59 patients) or foraminal stenosis (FS, 42 patients). After surgery, the patients were encouraged to ambulate and were discharged 2–3 days later without the use of a neck collar. Events occurring during hospitalization were documented in the hospital information system. Patients were followed-up for a mean period of 21±26 months (range, 1–110 months).
Results Clinical parameters improved from 1 month postoperatively and were maintained throughout the follow-up period, with no significant differences between the DH and FS groups (p>0.05). Complications occurred in 14 patients (14%) with no significant difference between the DH (8 of 59, 14%) and FS (6 of 42, 14%) groups (p>0.05). The most common complication was dural tear, followed by motor weakness, sensory changes, hematoma collection, incomplete decompression, reoperation, and wrong-level surgery. Two patients underwent reoperation due to symptomatic hematoma collection and symptom recurrence 3 years postoperatively.
INTRODUCTIONCervical spinal surgery is recommended for patients with cervical radiculopathy and cervical central stenosis when nonsurgical treatment is ineffective [1-4]. Current surgical options include anterior cervical discectomy fusion (ACDF), artificial disc replacement, posterior microforaminotomy, biportal endoscopic posterior foraminotomy, and posterior full-endoscopic cervical foraminotomy/discectomy (PECF) [4-11]. PECF is a full-endoscopic cervical spinal surgery technique [12]. While clinical outcomes do not differ significantly among these procedures, each has inherent advantages and limitations [13]. PECF offers several potential benefits, including minimal injury to posterior spinal structures, a lower incidence of adjacent segment disease relative to ACDF, and the ability to achieve similar clinical outcomes at lower medical costs than with ACDF [14,15]. The preservation of cervical motion without instrumentation may be another advantage of PECF [13,16]. However, like all surgical techniques, PECF carries a risk of complications. The most common concern is the disruption of spinal kinematics and subsequent reoperation due to injury to the facet joint [17,18]. Nevertheless, a systematic review by Zhang et al. [19] helped alleviate this concern by demonstrating that the reoperation rate was statistically similar between PECF and ACDF (1% and 3.9%, respectively). Recent studies have shown that cervical kinematics are not as heavily disrupted by PECF as they are by open foraminotomy [20-24]. Despite the minimally invasive nature of PECF, complications are possible, including suboptimal outcomes, hemorrhage, infection, injury to neural structures, loss of cervical lordosis, and subsequent progression to cervical kyphosis [16,17,25]. Therefore, this study was designed to analyze the complications following PECF at a single center and to present an up-to-date review of publications describing PECF complications.
MATERIALS AND METHODS1. PatientsThis study was approved by Seoul National University College of Medicine/Seoul National University Hospital of the Institutional Review Board (IRB No. 2101-080-1187). After receiving IRB approval, we conducted a retrospective review of patients who underwent PECF at a single institution between June 2010 and September 2022. The requirement for informed consent was waived by IRB for this retrospective study, as it posed no more than minimal risk and would not negatively impact the rights and welfare of the participants. This study included patients with (1) single- or dual-level unilateral radiculopathy due to cervical disc herniation (DH) or foraminal stenosis (FS), (2) a positive Spurling test, (3) disc space narrowing of no more than 50% [26], (4) complete preoperative clinical and radiological data, and (5) postoperative follow-up for more than 1 month [10]. Patients were excluded if they had (1) prior cervical spinal surgery; (2) malignancy, inflammatory joint disease, trauma, psychiatric disease, or neuromuscular disease; or (3) ossification of the posterior longitudinal ligament [10,21,25,27]. For DH cases, foraminal soft DH was confirmed using computed tomography (CT) and magnetic resonance imaging in the absence of evidence of bony FS. All patients with bony FS, as confirmed by CT and magnetic resonance imaging, were classified as having FS. In total, 101 patients (59 with DH and 42 with FS) were included in this study.
2. Surgical TechniquesThe surgical techniques for PECF were consistent with those previously reported [10,20-23,25,28-30]. PECF was performed with the patient in the prone position under general anesthesia (Figure 1). The surgical level was identified using C-arm fluoroscopy, and an 8-mm skin incision was made above the “V-point,” which is formed by the lamina, descending facet, and ascending facet [10,20-23,25,29,30]. A dilator (6.9-mm outer diameter), working channel (8.0-mm outer diameter), and endoscope (Vertebris, 4.1-mm working channel; Richard Wolf GmbH, Knittlingen, Germany) were sequentially introduced through the skin incision (Figure 2) [10,20-23,25,29,30]. Laminectomy and facetectomy were performed using an endoscopic drill under direct visualization. The size of bone drilling depended on the size and location of the herniated disc material and the extent of stenosis, typically within a radius of 3–4 mm around the V-point for soft DHs and 5–6 mm for FS [20-23,25]. Decompression and free movement of the nerve root were confirmed at the level of the shoulder/axilla and the superolateral/inferolateral corner of the nerve root (Figure 3) [10,20,22,25,29,30]. A closed-suction drain was inserted through the working tube, and the skin was closed (Figure 4). Patients were encouraged to walk on the day of surgery without a neck brace and were discharged the following day without limitations on neck motion [21,25].
3. Clinical EvaluationsAny events that occurred during hospitalization were documented in the hospital information system. Patient-reported outcome measures included the Neck Disability Index (scored out of 50) [31] as well as numerical rating scores for neck pain (NRS-N, out of 10) and arm pain (NRS-A, out of 10). These measures were evaluated before surgery and during outpatient clinic visits at 1, 3, 6, and 12 months postoperatively, as well as yearly thereafter. Patients were followed-up for an average of 21±26 months (range, 1–110 months).
4. Statistical AnalysisThe patients were divided into 2 groups: DH (n=59) and FS (n=42), with variables summarized as either mean (standard deviation) or frequency (proportion). The presence of any complications was assessed. Clinical outcomes were compared between the groups using the t-test at each time point. All analyses were performed using IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA). A 2-tailed p-value of less than 0.05 was considered to indicate statistical significance.
RESULTSThe most common surgical level was C6–7, followed by C5–6 (Table 1). In 99 patients, the procedure was single-level, while in 2 patients, it was 2-level. Clinical parameters demonstrated immediate improvement from 1 month postoperatively, and these improvements were sustained throughout the follow-up period (Table 2, Figure 5). No significant difference in clinical improvement was observed between the DH and FS groups (p>0.05). Complications arose in 14 patients (14%), with no significant difference in the complication rate between the DH group (8 of 59 patients, 14%) and the FS group (6 of 42 patients, 14%) (p>0.05) (Table 1). The most common complication was dural tear, followed by motor weakness, sensory changes, hematoma collection, incomplete decompression, reoperation, and wrong-level surgery (Table 1). Reoperation was performed in 2 patients due to symptomatic hematoma collection and symptom recurrence 3 years after surgery. Asymptomatic postoperative hematomas were closely monitored without sequelae. Although intraoperative dural tear occurred in 4 patients, the tears were minimal, and the arachnoid membrane remained intact. Consequently, the surgical wounds were closed without repairing the dura or applying an artificial dural patch. One patient experienced severe C6 nerve root palsy (Manual Muscle Testing grade 2 after surgery), likely due to an intraoperative bed hematoma. The hematoma was evacuated at the operative site, but motor weakness did not immediately resolve, and full recovery took 12 months. The other case of transient weakness (Manual Muscle Testing grade 4+ or 5) resolved within 1 month.
DISCUSSIONThe aim of this study was to examine complications following PECF and review the current literature on its complications. Our findings revealed a complication rate of 14%, with no significant difference between the DH and FS groups. These results suggest that the risk associated with the surgical procedure is similar across different pathologies.
1. Complications of PECFZhang et al. [19] conducted a systematic review indicating a 3% complication rate for PECF (95% confidence interval [CI], 1%–5%), which was lower than that of ACDF at 7.79% (95% CI, 5.54%–10.85%) (p<0.05). PECF has often been compared with endoscopy-assisted spinal surgery, specifically microendoscopic foraminotomy (MEF). In a separate systematic review, Wu et al. [32] revealed overall complication rates of 5.8% for PECF and 3.5% for MEF (p=0.12). Although these overall rates were similar between the procedures, transient root palsy was the most common complication after PECF (80%), while dural tear was the most common after MEF (42%) [32]. The rates of complications such as dural tear (PECF, 1.5%; MEF, 1.8%; p=0.67) and superficial wound infection (PECF, 2.2%; MEF, 1.0%; p=0.11) were not significantly different between groups [32].
2. Suboptimal Clinical OutcomesA frequently expressed concern regarding PECF is the potential for insufficient decompression and suboptimal outcomes. This concern may be valid, given the limited surgical view and instruments available. As demonstrated in this study, insufficient decompression occurred in the early stages of PECF (in the years 2015 and 2017) for 2 patients; however, reoperation was not performed due to substantial symptom improvement. Recent advances in optics and surgical instruments have helped to address this concern. As shown in Figure 3, complete decompression of the nerve root is now achievable, as recommended in standard surgical techniques [8,33,34]. The present study revealed that clinical outcomes had significantly improved by postoperative month 1 and were maintained for 2 years. Lv et al. [35] conducted a systematic review and found that both PECF and MEF resulted in substantial improvements in clinical outcomes, with no differences between the surgical techniques. Zhang et al. [19] compared PECF and ACDF and found no significant differences in the improvement of clinical outcomes, such as pain and Neck Disability Index, between procedures. Lee et al. [29] analyzed the recovery of preoperative weakness following PECF. In patients with mild weakness, normalization rates were 48%, 81%, 90%, and 96% at postoperative months 1, 3, 6, and 12, respectively. In patients with severe weakness, the improvement rates were 50%, 71%, 83%, 88%, and 92%, while the normalization rates were 8%, 38%, 58%, 58%, and 63% at postoperative months 1, 3, 6, 12, and 24, respectively [29]. These findings support the possibility that sufficient decompression can be achieved with PECF.
3. ReoperationAnother concern was the higher reoperation rate after posterior foraminotomy relative to that of ACDF. Lubelski et al. [36] analyzed matched cohorts and reported a reoperation rate of 6.4% at the index level after posterior open cervical foraminotomy and 4.8% after ACDF during a 2-year postoperative follow-up (p=0.07). A systematic review in 2019 showed similar reoperation rates (3.9% vs. 6.9%) and complication rates (7.8% vs. 4%) between ACDF and minimally invasive posterior cervical foraminotomy [6]. Despite the lack of statistical significance, the higher reoperation rate after posterior foraminotomy has been a concern. The present study showed that secondary surgery was necessary for one patient (1%) at the index level. Zhang et al. [19] analyzed the reoperation rate after PECF in a systematic review and found that it was not significantly different between PECF (1%) and ACDF (3.9%). Although PECF is a minimally invasive surgical technique, it is not a regenerative treatment; thus, degeneration may naturally progress by 2 years postoperatively, as shown in this study. However, the incidence was significantly lower than that of ACDF, suggesting the benefit of a minimally invasive surgical technique [8,11,19,34,37]. Biportal endoscopic surgery has recently received attention due to the comfortable transition from open surgery to this new procedure. In the near future, the effect of minimally invasive biportal cervical endoscopic surgery may be compared with that of PECF in a prospective study [8,11,38-41].
4. Neurological InjuryZhang et al. [19] conducted a systematic review and found that transient paresthesia was the most common complication after PECF (9 of 486, 1.8%), but it resolved over time. In the present study, sensory changes occurred in 2 patients after surgery, but these symptoms were managed with pregabalin for 1 month. The causes of paresthesia were multifactorial, potentially resulting from surgical trauma, thermal injury, or secondary changes after decompression. Motor weakness after posterior foraminotomy was not an uncommon complication and also occurred after PECF. In a systematic review, motor weakness was observed in 7 of 486 patients (1.4%), while in the present study, it occurred in 2% of patients [19]. Zhang et al. [19] reported that minor motor weakness (found in 3 patients) recovered after 3 months, while severe motor weakness was found in 4 patients and improved after 12 months. Motor weakness typically occurred due to excessive retraction of the nerve root [42]. Additionally, although it was not emphasized, a dual nerve root was detected in 20% of patients during surgery (Figure 6) [23,43]. The relative location of the nerve root over the disc space (the axilla of the nerve root in the lower cervical spine and the shoulder of the nerve root in the upper cervical spine) and the presence of dual roots should be considered to minimize nerve root injury [42]. A systematic review stated that dural injury occurred in 2 of 486 patients (0.4%), even though it was not a major focus of the study [19]. Uncertainty exists regarding whether repairing a torn dura is necessary during PECF, given the limited surgical instruments available for dural repair. In the current study, dural tear was the most common complication, but no patient required a second operation due to problems associated with cerebrospinal fluid leakage. Although the evidence is not robust, during PECF surgery, muscle is not resected but rather is split. After the removal of the working channel at the end of surgery, the muscles close by themselves [44]. This self-closure of the muscles may prevent cerebrospinal fluid leakage through the surgical wound. Another issue is intracranial hypotension, but this did not occur in the present study, possibly due to the space being too small to cause intracranial hypotension. However, a small amount of hematoma collection may cause neurological injury, as demonstrated in this study. Zhang et al. [19] showed that hematoma collection occurred in 2 of 486 (0.4%) patients after PECF in a systematic review. Therefore, closed-suction drainage may be helpful in preventing the collection of symptomatic hematoma at the surgical site (Figure 4), if necessary. To prevent neurological injury, careful manipulation of neural tissue, judicious use of surgical instruments and coagulators around neural tissue, and the insertion of closed suction, if necessary, are required [45].
5. Intraoperative SeizureAlthough increased intracranial pressure was not emphasized in a systematic review or the previous literature, unnoticed elevated epidural pressure may have catastrophic consequences [46]. While not reported during PECF, including in the present study, seizures have been reported in 3 of 816 patients (0.34%) during fully endoscopic lumbar surgery [46]. PECF is performed in water to create a surgical space and to wash out blood and surgical debris. These factors contribute to the advantages of PECF in minimizing soft tissue injury and postoperative infection, but the issue of water pressure remains. Increased epidural pressure may directly or indirectly damage the spinal cord or cause intracranial hypertension [46,47]. Joh et al. [48] demonstrated that indirectly transmitted increased epidural pressure from the lumbar spine to the cervical spine elicited neck pain, with the pressure at the neck averaging 53 mmHg (721 mmH2O). Although the exact mechanism of seizures during fully endoscopic spine surgery is still undetermined, factors such as infusion fluid containing cefazolin, infusion rate, prolonged operative time, dural tear, and sevoflurane anesthesia may increase the risk of seizures [46]. Symptoms and signs, such as headache, neck pain, seizures, elevated blood pressure, or bradycardia, should be carefully monitored in patients [49,50]. Lin et al. [46] reported the cases of seizures during full-endoscopic lumbar surgery and found that a so-called red flag sign—characterized by uncontrollable hypertension combined with a decreasing pulse rate—occurred in all 3 patients who experienced a seizure. Although not definitively established, this phenomenon bears similarity to the Cushing reflex, a cardiovascular response to compensate for increased intracranial pressure. This reflex sometimes occurs during endoscopic brain surgery, wherein the working space inside the brain is maintained with infused water pressure [49,50]. Previously, when a dural tear occurred during fully endoscopic spine surgery, 3 of 15 patients experienced seizures, and 1 of the 3 patients exhibited intracranial air on a postoperative CT scan [50]. Thus, strict control of epidural pressure is required when a dural tear occurs during surgery. The water pressure through the endoscopic system should be kept below 70 cmH2O by irrigating with saline using gravity or via careful use of a water irrigation pump to prevent increased intracranial pressure and unexpected intraoperative seizures [48,49].
6. Vertebral Artery InjuryDue to the proximity of the vertebral artery to the neural foramen, the artery may be injured during surgery. While no research is available on this specific complication, the authors have observed several cases of vertebral artery injury and subsequent infarction in the cerebellum and/or medulla oblongata at academic conferences. During the surgical procedure, a flexible coagulator may inadvertently enter the vertebral foramen (Figure 7), and an unnoticed injury caused by compression, coagulation, or vascular spasm may result in a vascular accident.
7. Radiological ChangesJagannathan et al. examined the segmental and cervical angles following posterior open cervical foraminotomy, finding a loss of cervical lordosis in 20% of patients (30 of 162), with one-third of these patients experiencing symptoms [17]. Despite this limitation, posterior cervical foraminotomy has been widely accepted as a valid surgical procedure for patients with radiculopathy, demonstrating a similar reoperation rate to that of anterior cervical discectomy and fusion [5,6,9,11,13,17,19,33,36,43,51,52]. PECF has recently emerged as an alternative to microscopic surgery, displaying comparable clinical outcomes in randomized controlled trials and systematic reviews [5,6,9,11,13,17,19,23,33,36,43,51,52]. The primary advantage of PECF lies in its minimally invasive nature, which can be attributed to the high magnification and illumination [25]. The resection of the facet joint involved removal of less than 10% of the joint’s original size (Figure 8) [25]. As a result, these benefits were evident in the improved cervical lordosis observed after PECF, even in patients with cervical hypolordosis [20,21], as well as in the preservation of cervical kinematics [10,22].
8. LimitationsWhile we attempted to address various reported and potential complications of PECF in this study, we acknowledge its limitations. First, the sample size was not large enough to establish a generalized consensus. The incidence of complications depends on each surgeon’s surgical technique and expertise. Second, this study was impacted by selection bias, as it did not include patients with severe cervical degeneration. The unique characteristics of severe degeneration, such as hypertrophied facet joints and perineural adhesion, were not considered in this study. These factors may have influenced the outcomes, including complications. Third, although this study involved a review of previous literature, it was not a systematic review. Finally, this study did not address long-term complications other than reoperation. The long-term effects of PECF on cervical degeneration and kinematics must be examined to improve surgical techniques. Despite these limitations, we have endeavored to discuss all types of reported and unreported complications of PECF in this manuscript. This information may be helpful in reducing complications associated with PECF.
NOTESFigure 1.Patient positioning. Surgery is performed with the patient in the prone position under general anesthesia. Gardner-Well tong skeletal fixation is utilized to facilitate the procedure. Careful attention is paid to ensure that the abdomen can freely sag, as this is important to reduce epidural venous congestion and bleeding. 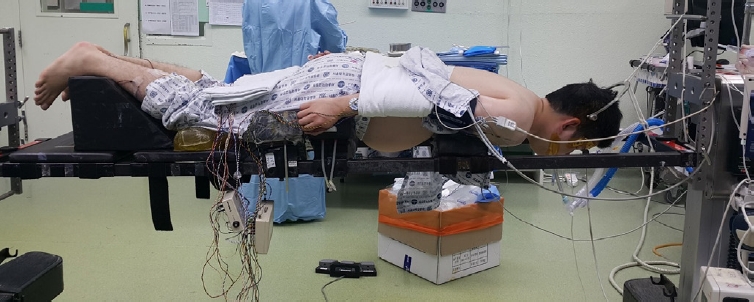
Figure 2.Surgeon’s working position. After introducing the spinal endoscope through an 8-mm skin incision, the surgeon holds the endoscopic system. The grip posture is discretionary, but to minimize fatigue during surgery, the arm should not be raised above the shoulder. 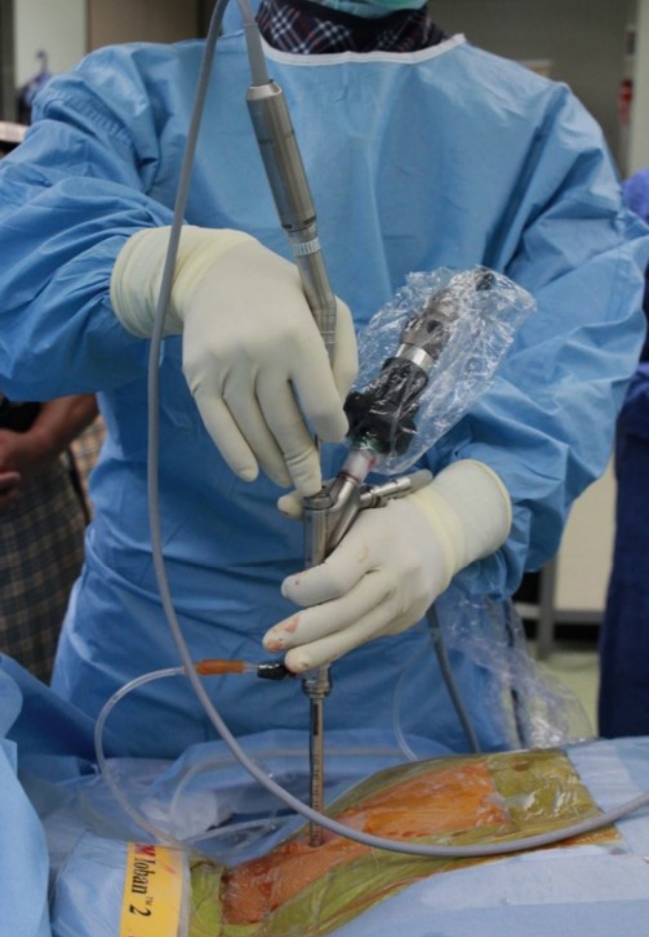
Figure 3.Nerve root decompression. Intraoperative photo demonstrates a decompressed C6 nerve root. Decompression and unimpeded motility of the nerve root are confirmed at the level of the shoulder/axilla (indicated by the yellow/white arrow) and the superolateral/inferolateral corner (marked by the red arrow) of the nerve root. 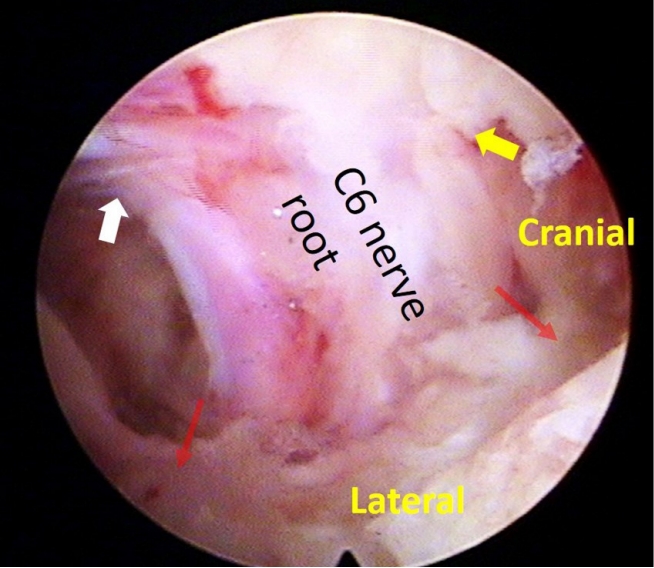
Figure 4.Closed-suction drain placement. A silastic drain is inserted through the working channel of the endoscopic system and is typically removed on postoperative day 1. 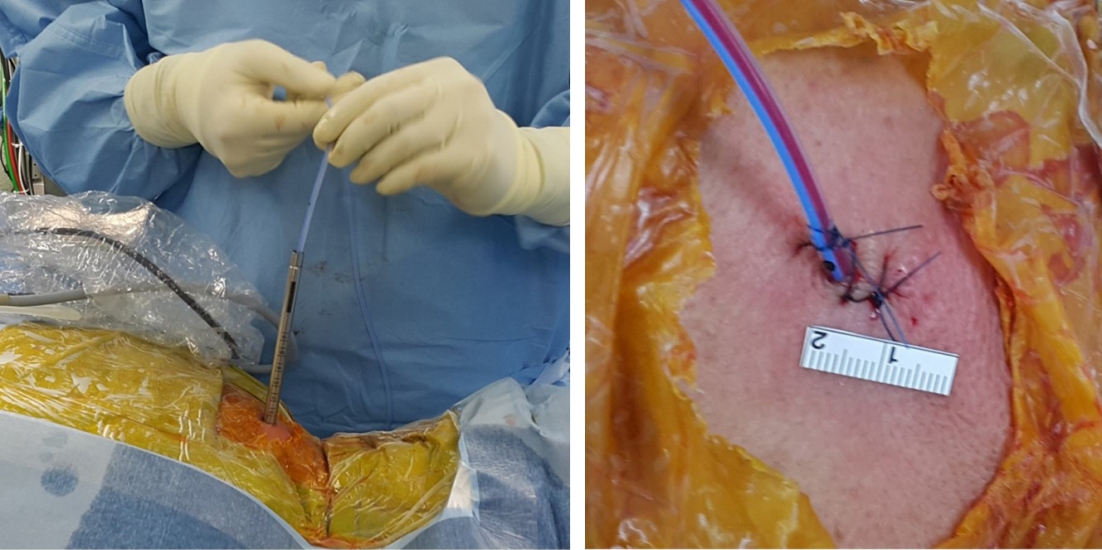
Figure 5.Clinical outcomes. By 1 month postoperatively, significant improvements were demonstrated in the Neck Disability Index (NDI, scored out of 50) (A), Numeric Rating Scale for neck pain (NRS-N) (B), and Numeric Rating Scale for arm pain (NRS-A) (C), with these improvements sustained throughout the follow-up period. No significant difference was observed between the disc herniation (DH) and foraminal stenosis (FS) groups. An asterisk (*) indicates statistical significance (p<0.05). 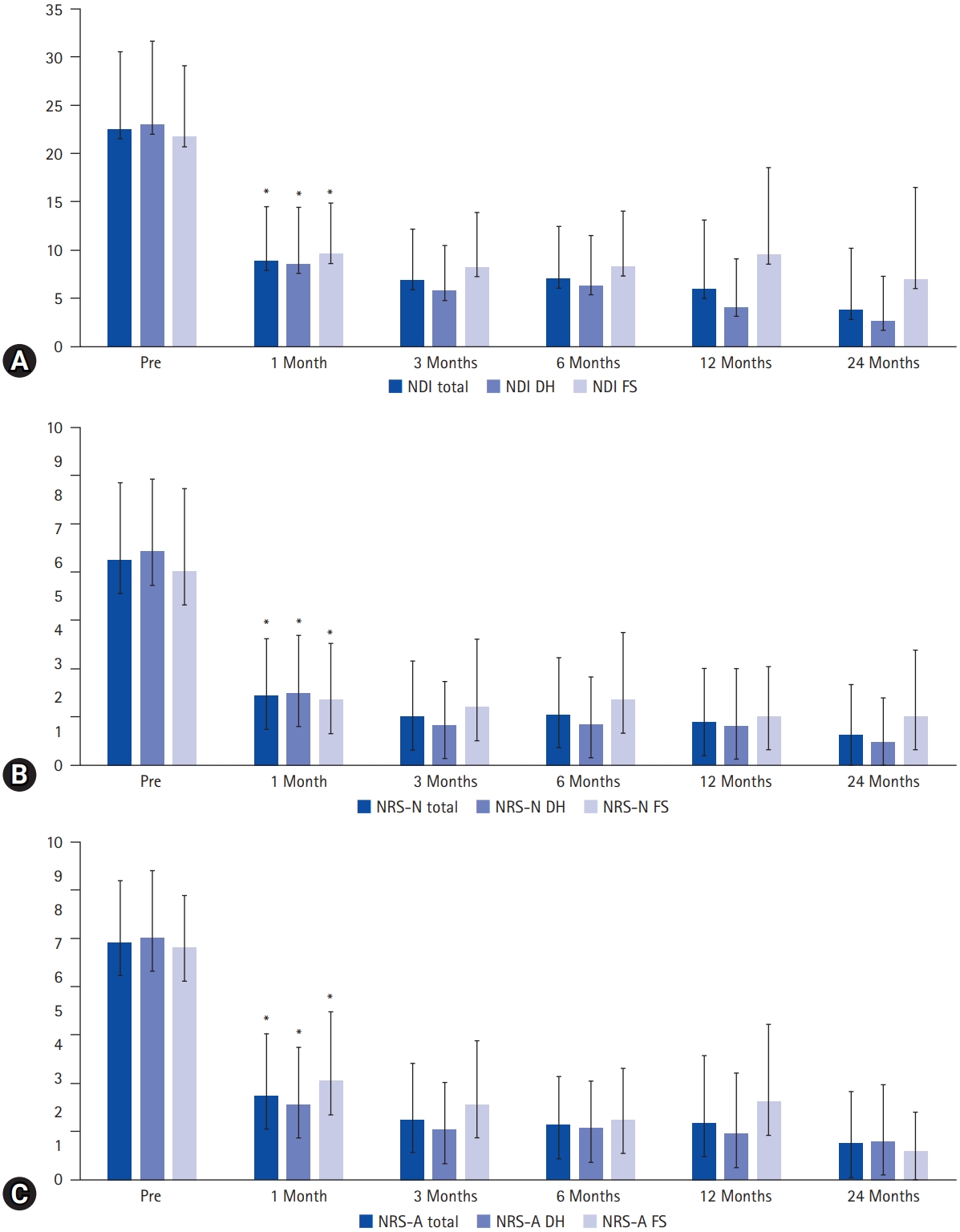
Figure 6.Dual nerve root. The ventral motor root is visible after retraction of the dorsal sensory root. 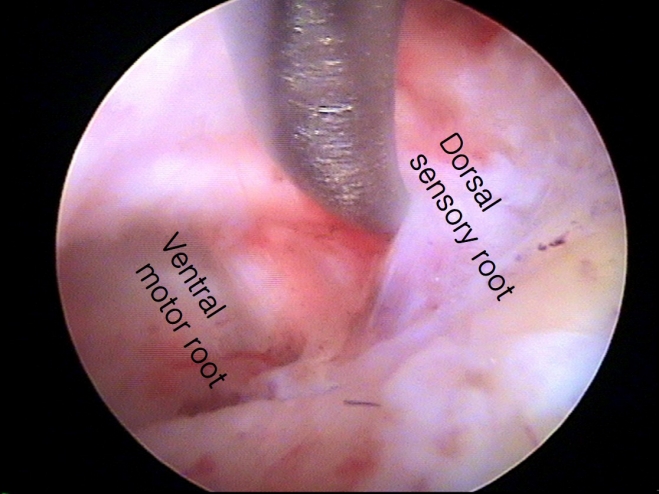
Figure 7.Potential risk of vertebral artery injury. Passage of the flexible electrode through the foramen to reach the vertebral artery, situated between the vertebral foramina, can result in vertebral artery injury. 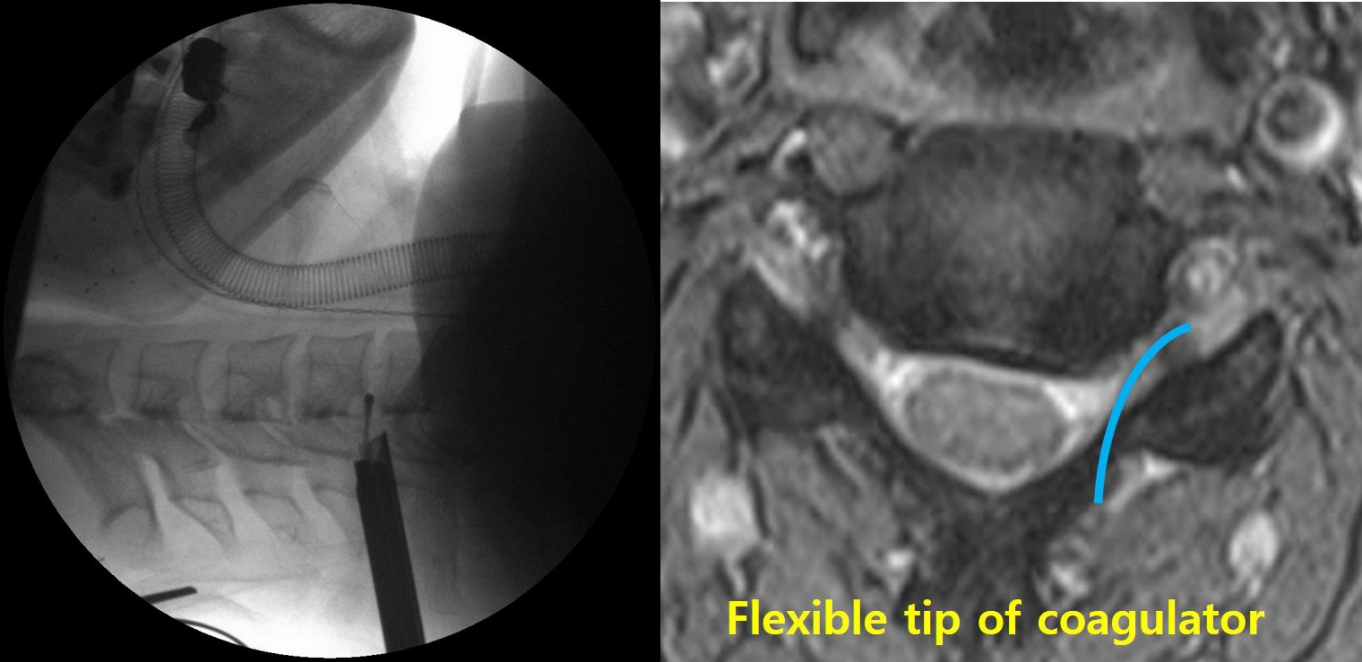
Figure 8.Postoperative computed tomography illustrates the typical extent of endoscopic foraminotomy.
The facet joint resection involves removing less than 10% of the joint’s original size. Blue arrow: site and extent of cervical endoscopic foraminotomy.
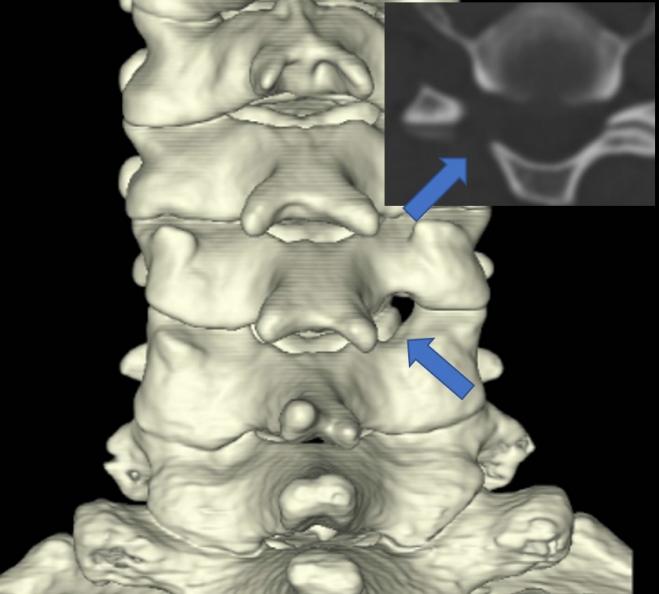
Table 1.Characteristics of patients Values are presented as mean±standard deviation or number. DH, disc herniation; FS, foraminal stenosis; NDI, neck disability index; NRS-N, Numerical Rating Scale for neck pain; NRS-A, Numerical Rating Scale for arm pain. Age: p=0.004 (There was a statistically significant age difference between patients who suffered from disc herniation and those that suffered from foraminal stenosis). *Two-level surgery. †A sensory complication refers to subjective deterioration of paresthesia/hypoesthesia after surgery. A motor complication refers to subjective and clinical deterioration of motor power, assessed with the Manual Muscle Testing grade scale. ‡Counted for each event in one patient. Table 2.Clinical outcomes REFERENCES1. Birkenmaier C, Komp M, Leu HF, Wegener B, Ruetten S. The current state of endoscopic disc surgery: review of controlled studies comparing full-endoscopic procedures for disc herniations to standard procedures. Pain Physician 2013;16:335–44.
2. Engel A, King W, MacVicar J; Standards Division of the International Spine Intervention Society. The effectiveness and risks of fluoroscopically guided cervical transforaminal injections of steroids: a systematic review with comprehensive analysis of the published data. Pain Med 2014;15:386–402.
3. Kim HJ, Kang MS, Lee SH, Park CH, Chung SW, Shin YH, et al. Feasibility of posterior cervical foraminotomy for adjacent segmental disease after anterior cervical fusion. J Korean Neurosurg Soc 2020;63:767–76.
4. Mok JK, Vaishnav AS, Chaudhary C, Alluri RK, Lee R, Urakawa H, et al. Impact of nonlordotic sagittal alignment on short-term outcomes of cervical disc replacement. Neurospine 2020;17:588–602.
5. Wu PF, Li YW, Wang B, Jiang B, Tu ZM, Lv GH. Posterior cervical foraminotomy via full-endoscopic versus microendoscopic approach for radiculopathy: a systematic review and meta-analysis. Pain Physician 2019;22:41–52.
6. Sahai N, Changoor S, Dunn CJ, Sinha K, Hwang KS, Faloon M, et al. Minimally invasive posterior cervical foraminotomy as an alternative to anterior cervical discectomy and fusion for unilateral cervical radiculopathy: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2019;44:1731–9.
7. Lee S, Cho DC, Chon H, Roh SW, Choi I, Park JH. Comparison between anterior cervical decompression with fusion and posterior cervical fusion with wide facetectomy for treatment of severe bony foraminal stenosis. J Korean Neurosurg Soc 2021;64:552–61.
8. Heo DH, Ha JS, Jang JW. Biportal endoscopic posterior cervical foraminotomy for adjacent 2-level foraminal lesions using a single approach (sliding technique). Neurospine 2023;20:92–8.
9. Yuh WT, Kim M, Choi Y, Han J, Kim J, Kim T, et al. Nationwide sample data analysis of additional surgery rate after anterior or posterior cervical spinal surgery. Sci Rep 2023;13:6317.
10. Paik S, Choi Y, Chung CK, Won YI, Park SB, Yang SH, et al. Cervical kinematic change after posterior full-endoscopic cervical foraminotomy for disc herniation or foraminal stenosis. PLoS One 2023;18:e0281926.
11. Wu B, Yang L, Fu C, Zhuo Y, Feng X, Xiong H. Global trends and hotspots in endoscopic discectomy: a study based on bibliometric analysis. Neurospine 2022;19:1093–107.
12. Hofstetter CP, Ahn Y, Choi G, Gibson JNA, Ruetten S, Zhou Y, et al. AOSpine consensus paper on nomenclature for working-channel endoscopic spinal procedures. Global Spine J 2020;10(2 Suppl):111s–121s.
13. MacDowall A, Heary RF, Holy M, Lindhagen L, Olerud C. Posterior foraminotomy versus anterior decompression and fusion in patients with cervical degenerative disc disease with radiculopathy: up to 5 years of outcome from the national Swedish Spine Register. J Neurosurg Spine 2019 Nov 15:1–9. doi: 10.3171/2019.9.SPINE19787. [Epub].
14. Witiw CD, Smieliauskas F, O'Toole JE, Fehlings MG, Fessler RG. Comparison of anterior cervical discectomy and fusion to posterior cervical foraminotomy for cervical radiculopathy: utilization, costs, and adverse events 2003 to 2014. Neurosurgery 2019;84:413–20.
15. Liu WJ, Hu L, Chou PH, Wang JW, Kan WS. Comparison of anterior cervical discectomy and fusion versus posterior cervical foraminotomy in the treatment of cervical radiculopathy: a systematic review. Orthop Surg 2016;8:425–31.
16. Fehlings MG, Gray RJ. Posterior cervical foraminotomy for the treatment of cervical radiculopathy. J Neurosurg Spine 2009 10:343–4. author reply 344.
17. Jagannathan J, Sherman JH, Szabo T, Shaffrey CI, Jane JA. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: a single-surgeon experience with a minimum of 5 years' clinical and radiographic follow-up. J Neurosurg Spine 2009;10:347–56.
18. Kim JY, Kim DH, Lee YJ, Jeon JB, Choi SY, Kim HS, et al. Anatomical importance between neural structure and bony landmark: clinical importance for posterior endoscopic cervical foraminotomy. Neurospine 2021;18:139–46.
19. Zhang Y, Ouyang Z, Wang W. Percutaneous endoscopic cervical foraminotomy as a new treatment for cervical radiculopathy: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e22744.
20. Won S, Kim CH, Chung CK, Choi Y, Park SB, Moon JH, et al. Comparison of cervical sagittal alignment and kinematics after posterior full-endoscopic cervical foraminotomy and discectomy according to preoperative cervical alignment. Pain Physician 2017;20:77–87.
21. Kim CH, Shin KH, Chung CK, Park SB, Kim JH. Changes in cervical sagittal alignment after single-level posterior percutaneous endoscopic cervical diskectomy. Global Spine J 2015;5:31–8.
22. Kim CH, Park TH, Chung CK, Kim KT, Choi YH, Chung SW. Changes in cervical motion after cervical spinal motion preservation surgery. Acta Neurochir (Wien) 2018;160:397–404.
23. Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:940–8.
24. Zhang C, Wu J, Zheng W, Li C, Zhou Y. Posterior endoscopic cervical decompression: review and technical note. Neurospine 2020;17(Suppl 1):S74–80.
25. Kim CH, Kim KT, Chung CK, Park SB, Yang SH, Kim SM, et al. Minimally invasive cervical foraminotomy and diskectomy for laterally located soft disk herniation. Eur Spine J 2015;24:3005–12.
26. Ofiram E, Garvey TA, Schwender JD, Denis F, Perra JH, Transfeldt EE, et al. Cervical degenerative index: a new quantitative radiographic scoring system for cervical spondylosis with interobserver and intraobserver reliability testing. J Orthop Traumatol 2009;10:21–6.
27. Skovrlj B, Gologorsky Y, Haque R, Fessler RG, Qureshi SA. Complications, outcomes, and need for fusion after minimally invasive posterior cervical foraminotomy and microdiscectomy. Spine J 2014;14:2405–11.
28. Oh SW, Singh R, Adsul NM, Noh JH, Park JH, Kim HS, et al. Anatomical relationship of the vertebral artery with the lateral recess: clinical importance for posterior cervical foraminotomy. Neurospine 2019;16:34–40.
29. Lee U, Kim CH, Chung CK, Choi Y, Yang SH, Park SB, et al. The recovery of motor strength after posterior percutaneous endoscopic cervical foraminotomy and discectomy. World Neurosurg 2018;115:e532–8.
30. Kim CH, Chung CK, Lee U, Choi Y, Park SB, Jung JM, et al. Postoperative changes in moderate to severe nonspecific low back pain after cervical myelopathy surgery. World Neurosurg 2018;116:e429–35.
31. Lee H, Nicholson LL, Adams RD, Maher CG, Halaki M, Bae SS. Development and psychometric testing of Korean language versions of 4 neck pain and disability questionnaires. Spine (Phila Pa 1976) 2006;31:1841–5.
32. Wu PF, Liu BH, Wang B, Li YW, Dai YL, Qing YL, et al. Complications of full-endoscopic versus microendoscopic foraminotomy for cervical radiculopathy: a systematic review and meta-analysis. World Neurosurg 2018;114:217–27.
33. Riew KD, Cheng I, Pimenta L, Taylor B. Posterior cervical spine surgery for radiculopathy. Neurosurgery 2007;60(1 Supp1 1):S57–63.
34. Kim JY, Heo DH, Lee DC, Kim TH, Park CK. Comparative analysis with modified inclined technique for posterior endoscopic cervical foraminotomy in treating cervical osseous foraminal stenosis: radiological and midterm clinical outcomes. Neurospine 2022;19:603–15.
35. Lv J, Mei J, Feng X, Tian X, Sun L. Clinical efficacy and safety of posterior minimally invasive surgery in cervical spondylosis: a systematic review. J Orthop Surg Res 2022;17:389.
36. Lubelski D, Healy AT, Silverstein MP, Abdullah KG, Thompson NR, Riew KD, et al. Reoperation rates after anterior cervical discectomy and fusion versus posterior cervical foraminotomy: a propensity-matched analysis. Spine J 2015;15:1277–83.
37. Ahn Y. Anterior endoscopic cervical discectomy: surgical technique and literature review. Neurospine 2023;20:11–8.
38. Kwon H, Park JY. The role and future of endoscopic spine surgery: a narrative review. Neurospine 2023;20:43–55.
40. Pao JL. Biportal endoscopic transforaminal lumbar interbody fusion using double cages: surgical techniques and treatment outcomes. Neurospine 2023;20:80–91.
41. Xu J, Wang D, Liu J, Zhu C, Bao J, Gao W, et al. Learning curve and complications of unilateral biportal endoscopy: cumulative sum and risk-adjusted cumulative sum analysis. Neurospine 2022;19:792–804.
42. Tanaka N, Fujimoto Y, An HS, Ikuta Y, Yasuda M. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine (Phila Pa 1976) 2000;25:286–91.
43. Komp M, Oezdemir S, Hahn P, Ruetten S. Full-endoscopic posterior foraminotomy surgery for cervical disc herniations. Oper Orthop Traumatol 2018;30:13–24.
44. Kim CH, Chung CK. Endoscopic interlaminar lumbar discectomy with splitting of the ligament flavum under visual control. J Spinal Disord Tech 2012;25:210–7.
45. Heo DH, Park DY, Hong YH, Kim D, Kim JS. Temperature change of epidural space by radiofrequency use in biportal endoscopic lumbar surgery: safety evaluation of radiofrequency. Eur Spine J 2023;32:2769–75.
46. Lin CY, Chang CC, Tseng C, Chen YJ, Tsai CH, Lo YS, et al. Seizure after percutaneous endoscopic surgery-incidence, risk factors, prevention, and management. World Neurosurg 2020;138:411–7.
47. Kang MS, Park HJ, Hwang JH, Kim JE, Choi DJ, Chung HJ. Safety evaluation of biportal endoscopic lumbar discectomy: assessment of cervical epidural pressure during surgery. Spine (Phila Pa 1976) 2020;45:E1349–56.
48. Joh JY, Choi G, Kong BJ, Park HS, Lee SH, Chang SH. Comparative study of neck pain in relation to increase of cervical epidural pressure during percutaneous endoscopic lumbar discectomy. Spine (Phila Pa 1976) 2009;34:2033–8.
49. Kalmar AF, Van Aken J, Caemaert J, Mortier EP, Struys MM. Value of Cushing reflex as warning sign for brain ischaemia during neuroendoscopy. Br J Anaesth 2005;94:791–9.
50. Vargas RAA, Moscatelli M, Vaz de Lima M, Ramirez Leon JF, Lorio MP, Fiorelli RKA, et al. Clinical consequences of incidental durotomy during full-endoscopic lumbar decompression surgery in relation to intraoperative epidural pressure measurements. J Pers Med 2023;13:381.
|
|
||||||||||||||||||||||||||||||||||||||||||||